JOURNAL OF INFECTIOUS DISEASES & MICROBIOLOGY
Prevalence and Antibiotic Resistance Profile of Salmonella Spp. in Broiler Carcasses from Dominant Poultry Production Areas in Bhutan
Kinley Penjor1*, Monu Gurung2, Kamrul Islam3, Ricardo J4 and Soares Magalhaes4
1Bhutan Food and Drug Regulatory Authority, HQ, Thimphu, Bhutan
2Bhutan Food and Drug Authority, Zhemgang, Bhutan
3UQ Centre for Clinical Research, Faculty of Medicine, The University of Queensland, Herston, QLD, 4029, Australia
4UQ Spatial Epidemiology Laboratory, School of Veterinary Science, The University of Queensland, Gatton, 4343 QLD Australia
*Corresponding Author: Kinley Penjor, Bhutan Food and Drug Regulatory Authority, HQ, Thimphu, Bhutan.
| ReceivedApr 10, 2023 | RevisedJun 1, 2023 | AcceptedJun 5, 2023 | PublishedJun 19, 2023 |
Abstract
Background: Salmonella is an important zoonotic pathogen, and its infections are considered among the most commonly and widely distributed food-borne illness reported worldwide. Poultry products have been identified as important sources of Salmonella infection to humans. While there have been reports of high prevalence of Salmonella and multidrug resistance in imported chicken meat in Bhutan, the safety of nationally produced broiler meat with regards to Salmonella contamination is not known. In the absence of national surveillance of Salmonella in food animals in Bhutan, this study assessed the prevalence of Salmonella and its serotypes in broiler chicken carcass in the dominant poultry production areas of the country and determine its antibiotic susceptibility patterns.
Methods: A cross-sectional study was conducted in January to April 2016 in all five major commercial broiler meat producers and suppliers in Bhutan located in the Samphelling and Darla gewogs. Following a systematic random sampling method, 36 broiler carcasses were sampled from each of the five broiler commercial farms producing a total sample size of 180 samples. Salmonella was isolated and identified following the International Organization for Standardization methods (ISO 6579:2002). All Salmonella isolates were subjected to antibiotic susceptibility testing (AST) following the procedures of the Clinical and Laboratory Standards Institute (CLSI) method.
Results: The overall prevalence of Salmonella in broiler chicken meat samples was 12.78% (95% CI, 10.98-14.58). The prevalence of Salmonella spp. was higher in farms of the samphelling gewog compared to Darla gewog [14.81% (95% CI, 8.2.7-21.5) vs 9.72% (95% CI, 2.9-16.6) respectively]. Among 23 Salmonella isolates, the most prevalent serovar was Salmonella ser. Typhi (73.9%). The Salmonella isolates showed high sensitivity to gentamicin (73.9% of isolates) followed by streptomycin (56.5%) and ampicillin (47.8%). The highest proportion of resistance was noted to tetracycline (95.6%), trimethoprim (86.9%), amoxicillin (65.2%), and ampicillin (47.8%). Among 23 Salmonella isolates, 60.87% (n=14) were considered multidrug resistant and 7 patterns of multidrug resistance were discovered.
Conclusion: The estimates of Salmonella prevalence and multidrug resistance in nationally produced commercial broiler chicken carcasses from the two main broiler meat producing areas in Bhutan, raises significant public health concerns highlighting the need for an integrated national Salmonella surveillance program across the poultry market chain.
Keywords
Antibiotics; Antimicrobial resistance; Antimicrobial susceptibility test; Bhutan; Chicken carcass; Food-borne; Multidrug resistant; Prevalence; Salmonella
Background
Salmonellosis caused by non-typhoidal Salmonella enterica, Salmonella ser. Paratyphi and Salmonella ser. Typhi have been recognized as a globally important foodborne illness of public health and economic significance in both humans and livestock [1]. It was estimated that poultry products such as eggs and fresh meat are the most implicated animal products linked to human Salmonellosis and have been identified as the main vehicles for Salmonella infection in humans [2].
Salmonellosis is the third leading foodborne cause of death worldwide behind norovirus (120 million cases) and Campylobacter spp. (96 million cases) and the leading foodborne illness (7.6 million cases) in terms of global disease burden (WHO, 2015). The global deaths from the food borne illness due to non-typhoidal Salmonella enterica accounted for 59,000 deaths of the total 2,30,000 deaths due to foodborne diarrheal disease agents (WHO, 2015). Likewise, the same study reported Salmonella Typhi as the major non-diarrheal causes of foodborne deaths (52000) globally (WHO, 2015). In 2010, the median global burden due to non-typhoidal Salmonella enterica was reported at 4,067,929 (95% CI, 2,486,092-6,271,290) Disability-Adjusted Live Years (DALYs) which translates to 78, 707, 591 (95%CI,31,843,647-211,154,682) illness and 59,153 (95% CI, 36,341-89,045) deaths. In the Southeast Asia subregion, the median burden due to non-typhoidal Salmonella enterica was reported at 1,042,715 (95%CI,225,416-2,824,443) DALYs which translates to 910 (95% CI, 89-4,760) DALYs per 100,000 population and ranked second behind African subregion. Similarly, the food borne burden due to Salmonella Typhi and Salmonella Paratyphi was reported at 250 illness and 58 illness per million population respectively in SEARO [3].
The study on the global burden of invasive nontyphoidal salmonellosis (iNTS) conducted in 2010, reported 3.4 million cases of iNTS per year which corresponds to 49 cases per 100,000 population.
In humans, the S. Typhi and S. Paratyphi cause typhoid fever and paratyphoid fever respectively and are transmitted through fecal oral route mainly via contaminated food and water [4].
The incubation period of typhoid fever is 10-14 days and causes a systematic febrile illness with prolonged low-grade fever, headache, nausea, anorexia, malaise, myalgia, a dry cough [5]. On other hand, the incubation period of paratyphoid fever is comparatively shorter with symptoms milder than typhoid fever. As per (Parry, 2002), about 10-15% of the typhoid cases develop severe to severe disease with common complication of typhoid encephalopathy, gastrointestinal bleeding, and intestinal perforation. In severe cases of typhoid fever, the mortality as high as 30-50% were reported from Indonesia and Papua New Guinea and reported a relapse of 5-10% of the cases which often follows antibiotic treatments [6].
Bhutan is largely an agrarian economy with 79% of its population engaged in agriculture and livestock farming. Approximately, 46% of households in Bhutan rear poultry for producing eggs and chicken (NSB, 2017). Since 2013, Bhutan has achieved 100% self-sufficiency in egg production. Yet, Bhutan still imports large volumes of chicken meat primarily from India to meet national consumer demand. In 2018, Bhutan imported more than 1500MT of chicken meat to meet the national consumer demand (RSD, 2018). Reports from Bhutan’s neighboring countries Bangladesh and India have reported increasing outbreaks of multidrug-resistant Salmonella strains as the result of consumption of poultry and their products in humans [7].
To reduce the incidence of salmonellosis in poultry, antibiotics are used either for disease prophylaxis or for the treatment of sick poultry [8]. In Bhutan legislation prohibits the use of antibiotics in feed as a growth promoter. The Drug Regulatory Authority (DRA), and Bhutan Agriculture & Food Regulatory Authority (BAFRA), Bhutan carries out regular inspections and monitoring of the feed plant to ensure antibiotics are not used by the feed manufacturers. Vaccines and antibiotics for poultry diseases are administered by livestock extension workers and are given free of cost by the government. However, anecdotal reports indicate that poultry farmers in bordering regions have access to antibiotics across the border and use them as prophylactics.
A study conducted in 2007 in imported chicken carcasses in Bhutan reported 13% prevalence of Salmonella with Salmonella ser. Enteritidis as the most prevalent serotype (84.62%) compared to Salmonella Typhimurium (15.38%) [9].
Based on the findings of this study, new provision was added to the legislation which requires importers to mandatorily produce sanitary certificates from the Export Inspection Council (EIC) in India certifying that chicken meat is safe and good quality.
While a regulatory framework for the control and prevention of Salmonellosis in imported poultry meat into Bhutan is presently enforced, the safety of locally produced chicken meat remains a public health concern as no studies have been conducted to establish Salmonella prevalence in nationally produced poultry.
Evidence on the epidemiology of Salmonella in domestically produced chicken meat in Bhutan, including data on antibiotic resistance patterns of Salmonella isolates, will help improve the understanding of occurrence and burden of Salmonellosis at food animal level and subsequently leverage the need for routine monitoring and surveillance in poultry.
This study was carried out to assess the prevalence of Salmonella and its serotypes in broiler chicken meat in high poultry production areas in Bhutan and investigate antibiotic sensitivity profiles of recovered Salmonella poultry serotypes. The findings will help inform the national Salmonella control plan to avert the public health burden associated with Salmonella infection.
Results
Farm and region level prevalence estimates of Salmonella contamination of broiler carcasses. Out of a total of 180 samples examined, the prevalence of Salmonella in broiler meat from the two major areas of broiler meat producer and supplier in Bhutan was 12.78% (95% CI, 10.98-14.58). The difference in prevalence of Salmonella spp. between Samphelling gewog and Darla gewog was 5.09 % (95% CL, 1.66-8.52).
The prevalence of Salmonella spp. in Samphelling gewog 14.81% (95% CI, 8.2.7-21.5) was higher than in Darla gewog 9.72% (95% CI, 2.9-16.6). Similarly, between the farms sampled, Salmonella prevalence was the highest in farm C 19.44% (7 isolates) followed by farm A 13.89% (5 isolates) and farm B and D 11.1% (4 isolates) and farm E 8.3% (3 isolates) (Table 1).
Farm C had one of the lowest biosecurity scores (14), one of lowest population sizes (2,050 head of poultry), was relatively recent operation (4 years) and imports DOC from India. There were no apparent relationships between farm-level seroprevalence and biosecurity indicators (Table 1).
Among the 23 Salmonella isolates, two Salmonella serovars were identified. The Salmonella Typhi 73.9% (17 isolates) serovar was found more predominant than Salmonella Paratyphi B 26.1% (6 isolates). Both the Salmonella serovars were prevalent in all farms in the study areas except for farm A (Table 1).
Antibiotic susceptibility profile of Salmonella isolates
These results demonstrate that the majority of Salmonella isolates were found sensitive to gentamycin 73.91% (17/23 isolates) and streptomycin 56.52% (13/23 isolates) (Table 2).
On the contrary, Salmonella isolates showed high resistance to tetracyclines 95.65% (22/23 isolates), trimethoprim 86.96 (20/23 isolates) and amoxicillin 65.20 (15/23 isolates). Overall, 60.87% (14/23 isolates) of the 23 isolates were considered multidrug resistant and 7 patterns of multidrug resistance were established.
About 95.65% (22/23 isolates) of the Salmonella isolates showed resistance to one or more antibiotics as shown in Figure 1. Both S. Typhi and S. Paratyphi B showed 94.1% (16/23 isolates) and 100% (isolates) resistance to one or more antibiotics respectively.
|
Prevalence |
Salmonella Serovar |
Resistance pattern |
Total biosecurity score |
Farm size |
Number of years in operation |
Disinfectant use |
Source of poultry feed |
Source of DOCs |
|||
|
S. Typhi (%) |
S. Paratyphi B (%) |
||||||||||
|
Farm A |
36 |
13.89 |
5(13.9) |
- |
2 |
16 |
2100 |
7 |
Bleaching Powder |
India |
Bhutan |
|
Farm B |
36 |
11.1 |
3 (8.3) |
1(2.7) |
3 |
14 |
2000 |
1 |
Bleaching Powder |
Feed plant |
Bhutan |
|
Farm C |
36 |
19.44 |
4(11.1) |
3(8.3) |
3 |
14 |
2050 |
4 |
Bleaching Powder |
Feed plant |
India |
|
Farm D |
36 |
11.1 |
3 (8.3) |
1(2.7) |
2 |
16 |
2000 |
7 |
KM04 |
Feed plant |
Bhutan |
|
Farm E |
36 |
8.3 |
2(5.5) |
1(2.7) |
2 |
15 |
2550 |
9 |
Bleaching Powder |
Feed plant |
India |
Table 1: Relationship between farm-level salmonella prevalence, serovars and resistance profiles and farm-level biosecurity indicator.
|
Antimicrobial agent |
No. of isolates tested |
Antibiogram pattern of Salmonella Spp. |
||
|
Resistant (%) |
Intermediate (%) |
Susceptible (%) |
||
|
Amoxicillin |
23 |
15(65.2) |
- |
8(38.5) |
|
Ampicillin |
23 |
11(47.8) |
1(4.4) |
11(47.8) |
|
Gentamicin |
23 |
3(13) |
3(13) |
17(74) |
|
Streptomycin |
23 |
2(8.7) |
8(34.8) |
13(56.5) |
|
Tetracycline |
23 |
22(95.7) |
1(4.3) |
- |
|
Trimethoprim |
23 |
20(87) |
- |
3(13) |
Table 2: Antimicrobial susceptibility pattern of Salmonella spp. isolated from raw broiler meat samples tested by disc diffusion method.
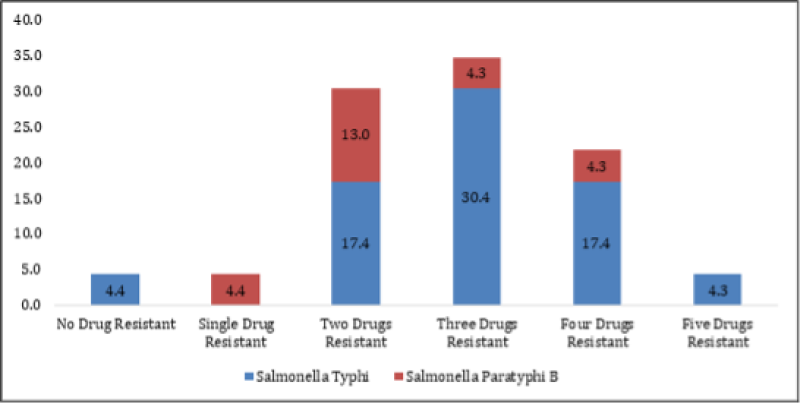
Figure 1: Percentage Resistance of Salmonella serovar recovered from raw broiler meat to six antimicrobial drugs.
Discussion
This research improves the evidence base on Salmonella epidemiology in commercially produced poultry in Bhutan by estimating the overall prevalence of Salmonella in broiler meat from the two major producer and supplier of broiler meat areas in Bhutan. Apart from this study only two other studies were available in the published literature from Bhutan on Salmonella in the animal sector: one in imported chicken meat and another in nationally produced beef.
This investigation identified two Salmonella serovars, S. Typhi 17 (73.9%) and S. Paratyphi B 6 (26.1%) and that among 23 Salmonella isolates, 60.87% were multidrug resistant and as high as 6 patterns of multidrug resistance were recorded. The detection of Salmonella in nationally produced broiler meat and the presentation of high proportion of multidrug resistant Salmonella indicates the need to further investments into the farm to fork approach to Salmonella control in Bhutan.
The Salmonella prevalence in raw broiler meat estimated in this study (13%) is comparable to that of Salmonella prevalence in imported chicken meat in Bhutan and to that reported prevalence of Salmonella in beef in Bhutan (15.8%). These results are also in line with studies conducted in Japan and in Egypt. Where a prevalence of 12.2% was reported in retail chicken products and broiler chicken respectively. However, these results are much lower to studies reported from other countries in the South Asian region, where the prevalence of Salmonella was estimated as high as 65.5% in frozen chicken meat and 38.7% in broiler farm in Bangladesh, 33.3% in chicken carcass. In India, and 57% in chicken meat in Nepal were reported. Also, in Vietnam and China, the prevalence of Salmonella in chicken carcasses was (48.7%) and 43.3% respectively. The varying prevalence of Salmonella in chicken meat between sampled commercial farms could be attributed to varying on-farm biosecurity measures applied and hygiene practices at the production system. Other studies have shown that cleaning equipment and disposing of dead birds as significant risk factors for Salmonella detection in farms. The study concluded that the variation of prevalence of Salmonella between farms could be attributed to the farm chicken breed.
Differences in the prevalence between the study farms can be attributed to differences in bird density as higher bird density has been noted in previous studies to be associated with increased odds of detection of positive Salmonella [10]. In Bhutan, commercial poultry (broiler and layer) are reared in deep litter production system and depending on the size of the farms, farmers usually have more than two poultry shed, and each shed has the capacity of more than 1000 birds. All five commercials’ farms had farm sizes between 2,000-2,500 birds and follow all-in all-out system.
The source of poultry could also pose a biosecurity risk for the introduction of Salmonella into a flock. In this study, broiler day old chicks used by these farms were generally sourced within the country and supplied by the National Poultry Research and Development Canter (NPRDC) which is run by the Department of Livestock under the Ministry of Agriculture & Forest, Bhutan. However, during periods of national shortage of DOCs, farmers resort to importing DOCs directly from hatcheries in India which are registered with BAFRA, Bhutan. The majority of the poultry farmers use feeds which are manufactured within the country; however, few poultry farmers procure feed directly from India. As per the farm biosecurity survey conducted by BAFRA in 2018, the poultry commercial farms had good farm biosecurity practices with clean water source, proper rodent control, proper fencing, proper documentation of visitor, treatment, feed, and vaccination. In Bhutan, commercial broiler farms have a designated slaughter facility at the farm. The slaughter facility has minimum equipment for sticking, defeathering, evisceration and are carried out in same shed but separated by partition wall to avoid cross contamination.
In addition, to husbandry systems other factors could be implicated in the variation in prevalence observed in this study. As per [11]. The difference between Salmonella prevalence in this study and others reported in the literature could be due to difference in the study design particularly number of samples collected, sample collection period, sample type, sampling procedures and method of detection used. Studies conducted in China, Greece and the USA described that prevalence and concentration of Salmonella in chicken was relatively higher in summer and spring than winter and autumn.
The two Salmonella serovars detected in this study were S. Typhi and S. Paratyphi B. Among the two serovars, the S. Typhi was found to be more prevalent 73.9% (17 isolates) than S. Paratyphi B 26.1% (6 isolates).
S. Typhi and S. Paratyphi B were also recovered from chicken carcass in Egypt, Indonesia and India broiler farm in Bangladesh and eggs in Nepal. Both the Salmonella serovars fall in typhoid subcategory of Salmonella meaning it infects a very narrow range of hosts including humans [12-15] and are usually associated with higher number of fatal cases. These serovars do not present poultry as a reservoir but rather an indication of contamination during the slaughter processes at different points of the production line. The predominant Salmonella serovar reported in Asia region were S. Typhimurium (15.34%) and S. Enteritidis (69.84%) in China in chicken meat, S. Typhimurium (15.38%) in Bhutan in imported chicken and S. Typhimurium (22.2%) in chicken meat in Nepal. A meta-analysis study on the worldwide epidemiology of Salmonella serovar in animal-based food reported S. Typhimurium as the most prevalent and disseminated serovar worldwide. The other serovar reported globally were S. Pullorum, S. Gallinarum, S. Enteritidis, and S. enteric in chicken [16].
These results also indicate a high proportion of Salmonella isolates were sensitive to gentamycin 73.91% (17 isolates) and streptomycin 56.52% (13 isolates) but high resistance to tetracyclines 95.65% (22 isolates), trimethoprim 86.96 (20 isolates) and amoxicillin 65.20 (15 isolates). This study findings were in consistent with the findings which labelled doxycycline, ampicillin, amoxicillin, and tetracycline resistant to Salmonella isolates but, sensitive to norfloxacin, enrofloxacin, gentamicin and ciprofloxacin. In the Asia region, high resistance of Salmonella was reported for tetracycline (97.14%) and chloramphenicol (94.28%), in chicken farm in Bangladesh trimethoprim sulfamethoxazole (70.3%) and tetracycline (54.3%) in chicken meat in Myanmar, sulphonamide compounds (98.9 %) and tetracycline (96.9 %) in chicken carcass in Nepal [17,18]. The emergence of high resistance of Salmonella to tetracycline, amoxicillin and trimethoprim antimicrobials in the study may be contributed to indiscriminate use of these antimicrobials in poultry production as growth promotion, prophylactic and therapeutic purposes [19] and Bhutan has no exception. As per the drug distribution report of Bhutan, 2018, the tetracycline trimethoprim was largely distributed for the poultry use. The consistent reports of high resistance of Salmonella to tetracycline, trimethoprim and amoxicillin antimicrobials in several studies conducted globally raise serious public health concern since these antimicrobials are used as first line drugs for human and animals. This study also observed that 95.65% (22 isolates) of the Salmonella isolates showed resistance to one or more antibiotics with six antibiotic resistance patterns and 60.87% of multidrug resistance. A study conducted by Dahal in imported frozen chicken in Bhutan, also reported Salmonella resistance to one or more drugs antimicrobials namely, nalidixic acid (96.15%), amoxicillin (11.54%) and cephalexin (5.77%) [17]. A widespread multidrug resistance of Salmonella was reported in chicken eggs in Nepal, broiler chicken at slaughterhouse in China and broiler farm in Bangladesh. Recognizing the role of meat in Salmonella transmission to humans added by reports of high prevalence of Salmonella and multidrug resistance from the two main producer and supplier of broiler meat areas in Bhutan, altogether, it raises serious public health concern which require immediate action.
The findings of this study need to be interpreted in light of the fact that within the five commercial broiler farms that fulfilled the study’s inclusion criteria, researchers collected samples from the same batch of birds in the farm to get the required sample size. While employed a cluster sampling weight to adjust the sample size calculation, birds in the batch are usually raised in same production system.
In this study present the first estimates of Salmonella prevalence and antibiotic resistance profiles in nationally produced broiler meat in the dominant broiler production and supply areas in Bhutan. These findings should serve as a baseline information and recommends for a systematic and comprehensive surveillance study at farm level, at slaughter processing line, and at retail meat shop to understand the source of Salmonella contamination and its prevalence including the antibiotic resistance profile.
Such comprehensive findings can be used to guide the policy marker in designing plans and programs for monitoring and control of Salmonella at the national level to help reduce the human risk of Salmonellosis at the country level.
Materials and Methods
Study setting and sampling frame
A cross-sectional study was conducted from January to April 2016 to assess the prevalence of Salmonella and its serotypes in broiler chicken meat in high poultry production areas in Bhutan and to investigate their antibiotic susceptibility profile. The two major areas that produce and supply commercial chicken broilers in the country-the gewogs of sampling and Darla located in the Chukha district-were selected for this investigation. The study focused on sampling chicken broilers produced in commercial poultry enterprises. For the purpose of the study, commercial chicken broiler farms were defined as farms which have more than 2,000 birds with a permanent shed. As per the sampling frame, there were a total of five commercial broiler farms in both the study areas fulfilling the commercial farm definition. All five commercial broiler farms (Farm A, B, C, D and E) were included in the study.
These enterprises were run by different farmers under different production management. Researchers collected farm biosecurity management data including number of years in operation, presence of perimeter fencing, presence of disinfection points, presence of biosecurity sign boards, presence of visitor logbook, source country of DOCs, vaccination and deworming protocols, mortality and treatment record, source of feed, treatment of water, presence of a bio pit, utilization of own farm equipment, use of PPE (ie. gloves, mouth covers, boots, and apron), presence of meat processing facility, cleaning, and disinfection protocols (animal shed, farm disinfection, disinfectant use, premise clean, drainage clean).
The information on farm biosecurity management was collected by the lab technicians using pretested questionnaires. The filing of questionnaires involved an interview, observations, and validation methods.
Sampling methods and procedures
The sample size for the prevalence survey was calculated using the epi tool formula: n= (Z² x P (1-P)/e² where Z: is the value from standard normal distribution corresponding to desired confidence level (for 95% CI), P: is expected true proportion, e: is desired precision. [At 95% CI, α: 5%] and sample size was calculated as n=180.
During the slaughter time poultry are pooled at the slaughter facility and are slaughtered by trained workers following hygienic practices. Depending on the market demand, the slaughter period of the bird will vary from farm to farm. Usually, the birds are slaughtered between 40-50 days of rearing from the day-old chick (DOC). Since all five farms in the study follow an all-in all-out system, only one production cycle of the broiler birds was considered and 36 samples each from one farm was collected in each visit.
The schedule of sample collection was tied to the slaughter date of the broilers in a farm and were collected over 5 weeks period (i.e., one visits every week).
Following a systematic random sampling method, 36 healthy broiler carcasses from each of the five broiler commercial farms in sampling and Darla gewogs were selected after the evisceration and before packaging resulting in a total sample size of 180 samples. From the selected broiler carcasses, a breast muscle portion weighing approximately 30g was collected in a sterile plastic sachet by the trained laboratory technicians. The samples were stored at below 4 °C temperature in cool box and were transported to the laboratory within 8 hours from the time of sample collection. During the sample collection time the additional information on farm size, farm biosecurity status, source of feed, water and DOC, and management practices was also collected.
Isolation and identification
Samples were analyzed at the National Food Testing Laboratory which is accredited to ISO/IEC 17025. For the culture of Salmonella, the International Organization for Standardization methods (ISO 6579:2002) was followed.
A finely cut pieces of 25 grams of chicken breast was mixed with 225 ml of buffered peptone water and was shaken for 2 minutes. The mixture was incubated at 37 °C ± 1 °C for 18 ± 2 hours. After the incubation period, a loop-full of material from the Rappaport-Vassiliadis soya (RVS) broth and Muller Kauffmann tetrathionate novobiocin (MKTTn) was transferred and streaked separately onto the Brilliant green Phenol Red Lactose Sucrose agar and Xylose lysine deoxycholate (XLD) agar separately and were incubated at 37 °C for 24 hours. The plates were incubated in an inverted position and observed for the growth of typical Salmonella colonies.
The combination of biochemical and serological tests was used for identification and confirmation of isolates of Salmonella. The pure colonies were subjected to six biochemical confirmation tests namely, Triple sugar agar (TSI agar), Urea agar, L-lysine decarboxylation medium, Voges-Proskauer reaction, Methyl red reaction, and indole reaction. After biochemical confirmation, all Salmonella positive isolates were transferred into half-strength NA in 1.5-mL Eppendorf tubes and stored at 48 °C. The pure colonies showing typical biochemical reactions for Salmonella were tested for the presence of Salmonella O antigen. However, research could not conduct further serotyping, molecular typing of isolates given that authors laboratory does not have these facilities available.
Antimicrobial susceptibility
Antibiotic sensitivity testing for identified Salmonella isolates was carried out using the disc diffusion method described in Clinical and Laboratory Standards Institute (CLSI, 2016). A total of six antimicrobials used in the disc diffusion method including Ampicillin (10mcg/disc), Amoxycillin (30 mcg/disc), Tetracycline (30mcg/disc), Streptomycin (10mcg/disc), Trimethoprim (5 mcg/disc) and Gentamicin (10 mcg/disc). These antimicrobials were selected since antimicrobials are commonly used in poultry industries in Bhutan. The results were interpreted using CLSI methods. As per the National Veterinary Drug Formulary 2016 (4th edition), amoxicillin trihydrate alone or in combination with colistin is approved for treatment of poultry in the country. Further, the laboratory did not have amoxicillin/clavulanic acid disc. Therefore, an amoxicillin disc alone is used for this study. The interpretation of amoxicillin disc diffusion was done using guidelines from clinical veterinary microbiology by J. Smith, 1995. The multidrug resistance for this study is defined as resistance to three or more antimicrobial classes [20].
Statistical analyses
Sample laboratory results and farm-level biosecurity data were entered into Microsoft Excel, version 2016. The data were then transferred into the WHONET offline database. Prevalence of Salmonella spp. was calculated from the total number of samples tested and the prevalence of Salmonella serovars was calculated from the total number of positive Salmonella spp. isolated. Confidence intervals for a single proportion and difference in proportions were estimated using the stats model’s library in python3.
The sensitivity and resistance to different antimicrobials of Salmonella spp. positive isolates were presented as a proportion. The epidemiological data analyses of antibiogram patterns were managed using WHONET which is an analytical tool that facilitates understanding of the local epidemiology of microbial populations and selection of antimicrobial agents [21].
Each farm-level biosecurity indicator was scored a value of “1” when the response represented an adequate biosecurity practice and “0” when the response was less adequate. A total farm-level biosecurity score was estimated as the sum of all responses to the questionnaire. Results were compared descriptively against estimates of prevalence.
|
Antimicrobial Resistance Patterns |
Commercial Broiler Farms |
||||
|
Farm A |
Farm B |
Farm C |
Farm D |
Farm E |
|
|
No_pattern |
- |
1 |
- |
- |
- |
|
TET_TMP |
2 |
- |
5 |
3 |
- |
|
TET_AMX |
- |
1 |
- |
- |
- |
|
TET_TMP_AMP |
3 |
- |
- |
- |
- |
|
TET_TMP_AMX |
- |
1 |
- |
- |
1 |
|
TET_TMP_AMX_AMP |
- |
1 |
1 |
- |
- |
|
TET_TMP_AMX_AMP_STR |
- |
- |
1 |
- |
2 |
|
TET_TMP_AMX_AMP_GEN |
- |
- |
- |
1 |
- |
Table 3: Antimicrobial resistance patterns for different farms.
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Availability of data and materials
The authors confirm that all the raw data supporting the findings of this study are available within the article and its supplementary materials.
Competing interests
The authors declare no competing interests.
Acknowledgements
The authors wish to thank the National Food Testing Laboratory, Yusipang Thimphu for helping in the collection of samples and for providing the facilities for carrying out this research work.
References
1. Westrell T, Ciampa N, Boelaert F, Helwigh B, Korsgaard H, Chríel M, et al. Zoonotic Infections in Europe in 2007: A Summary of the EFSA-ECDC Annual Report. Euro Surveill. 2009;14(3):19100. PubMed | CrossRef
2. Authority EF. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food‐Borne Outbreaks In 2017. EFSA J. 2018;16(12). PubMed | CrossRef
3. Havelaar AH, Kirk MD, Torgerson PR, Gibb HJ, Hald T, Lake RJ, et al. Bellinger Dc, De Silva Nr, Gargouri N, Speybroeck N. World Health Organization Global Estimates and Regional Comparisons of The Burden of Foodborne Disease In 2010. PLoS Med. 2015;12(12):e1001923. PubMed | CrossRef
4. Westrell T, Ciampa N, Boelaert F, Helwigh B, Korsgaard H, Chríel M, et al. Zoonotic Infections in Europe in 2007: A Summary of the EFSA-ECDC Annual Report. Euro Surveill. 2009;14(3):19100 PubMed | CrossRef
5. Zdragas A, Mazaraki K, Vafeas G, Giantzi V, Papadopoulos T, Ekateriniadou L. Prevalence, Seasonal Occurrence and Antimicrobial Resistance of Salmonella in Poultry Retail Products in Greece. Lett Appl Microbiol. 2012;55(4):308-13. PubMed | CrossRef
6. Bhan, MK, Bahl, Rajiv, Bhatnagar, Shinjini. Typhoid and Paratyphoid Fever. Lancet, 2005;366(9487):749-762 PubMed | CrossRef
7. Gotuzzo ED, Morris Jr JG, Benavente LU, Wood PK, Levine OR, Black RE, et al. Association Between Specific Plasmids and Relapse in Typhoid Fever. Lancet. 1987;25(9):1779-81 PubMed | CrossRef
8. Shrestha A, Bajracharya AM, Subedi H, Turha RS, Kafle S, Sharma S, et al. Multi-Drug Resistance and Extended Spectrum Beta Lactamase Producing Gram Negative Bacteria from Chicken Meat in Bharatpur Metropolitan, Nepal. BMC Res Notes. 2017;10(1):1-5. PubMed | CrossRef
9. Helke KL, McCrackin MA, Galloway AM, Poole AZ, Salgado CD, Marriott BP. Effects of Antimicrobial Use in Agricultural Animals on Drug-Resistant Foodborne Salmonellosis in Humans: A Systematic Literature Review. Crit Rev Food Sci Nutr. 2017;57(3):472-88. PubMed | CrossRef
10. Wang J, Wu H, Song M, Li F, Zhu J, Xi M, et al. Prevalence and Quantitative Detection of Salmonella in Retail Raw Chicken in Shaanxi. China. J Food Prot. 2013;76(11):1958-62. PubMed | CrossRef
11. Donado-Godoy P, Gardner I, Byrne BA, Leon M, Perez-Gutierrez E, Ovalle MV, et al. Prevalence, Risk Factors, And Antimicrobial Resistance Profiles of Salmonella from Commercial Broiler Farms in Two Important Poultry-Producing Regions of Colombia. J Food Prot. 2012;75(5):874-83. PubMed | CrossRef
12. Rodriguez A, Pangloli P, Richards HA, Mount JR, Draughon FA. Prevalence of Salmonella in Diverse Environmental Farm Samples. J Food Prot. 2006;69(11):2576-80. PubMed | CrossRef
13. Mridha D, Uddin MN, Alam B, Akhter AT, Islam SS, Islam MS, et al. Identification and Characterization of Salmonella Spp. From Samples of Broiler Farms in Selected Districts of Bangladesh. Vet World. 2020;13(2):275. PubMed | CrossRef
14. Hendriksen, Rene S, Vieira, Antonio R, Karlsmose, Susanne, et al. Global Monitoring of Salmonella Serovar Distribution from The World Health Organization Global Foodborne Infections Network Country Data Bank: Results of Quality Assured Laboratories from 2001 To 2007. Foodborne Pathog Dis. 2011;8(8):887-900 PubMed | CrossRef
15. Maka L, Popowska M. Antimicrobial Resistance of Salmonella Spp. Isolated from Food. Roczniki Państwowego. Rocz Panstw Zakl Hig. 2016;67(4). PubMed
16. Newell DG, Koopmans M, Verhoef L, Duizer E, Aidara-Kane A, Sprong H, e al. Food-Borne Diseases-The Challenges Of 20 Years Ago Still Persist While New Ones Continue to Emerge. Int J Food Microbiol. 2010;139:3-15. PubMed | CrossRef
17. Pires SM, De Knegt L, Hald T. Estimation of The Relative Contribution of Different Food and Animal Sources to Human Salmonella Infections in The European Union. EFSA Support Publ. 2011;8(8):184E. CrossRef
18. Murugkar HV, Rahman H, Kumar A, Bhattacharyya D. Isolation, phage typing & antibiogram of Salmonella from man & animals in northeastern India. Indian J Med Res. 2005;122(3):237. PubMed
19. Mridha D, Uddin MN, Alam B, Akhter AT, Islam SS, Islam MS, et al. Identification and Characterization of Salmonella Spp. From Samples of Broiler Farms in Selected Districts of Bangladesh. Vet World. 2020;13(2):275. PubMed | CrossRef
20. Falagas ME, Koletsi PK, Bliziotis IA. The Diversity of Definitions of Multidrug-Resistant (MDR) And Pandrug-Resistant (PDR) Acinetobacter Baumannii and Pseudomonas Aeruginosa. J Med Microbiol. 2006;55(12):1619-29. PubMed | CrossRef
21. Agarwal A, Kapila K, Kumar S. WHONET Software for The Surveillance of Antimicrobial Susceptibility. Med J Armed Forces India. 2009;65(3):264-6. PubMed | CrossRef
Kinley Penjor1*, Monu Gurung2, Kamrul Islam3, Ricardo J4 and Soares Magalhaes4
1Bhutan Food and Drug Regulatory Authority, HQ, Thimphu, Bhutan
2Bhutan Food and Drug Authority, Zhemgang, Bhutan
3UQ Centre for Clinical Research, Faculty of Medicine, The University of Queensland, Herston, QLD, 4029, Australia
4UQ Spatial Epidemiology Laboratory, School of Veterinary Science, The University of Queensland, Gatton, 4343 QLD Australia
*Corresponding Author: Kinley Penjor, Bhutan Food and Drug Regulatory Authority, HQ, Thimphu, Bhutan.
Copyright© 2023 by Penjor K, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Penjor K, Gurung M, Islam K, Ricardo J, Magalhaes S. Prevalence, And Antibiotic Resistance Profile of Salmonella Spp. In Broiler Carcasses from Dominant Poultry Production Areas in Bhutan. J Infect Dis Microbiol. 2022;1(3):12-24. DOI: https://doi.org/10.37191/Mapsci-JIDM-1(3)-013
