JOURNAL OF BIOMEDICAL AND ALLIED RESEARCH
Evaluation of Transplanted Stem Cell Dynamic Variables in the Bone Marrow of Children with Malignancies
Pınar Akpınar Oktar1*, Merve Güneş2, Rumeysa Kılıç3, Türkan Patıroğlu4, Üstün Ezer5 and Ahmet Emin Kürekçi6
1Dr. Biologist, Operational Director of Tissue Typing and Immunology Laboratory Lösante Hospital Kızılcaşar, 23 Nisan St. No:20, 06830 Gölbaşı/Ankara, Turkey
2MSc. Bioengineer, Tissue Typing and Immunology Laboratory LÖSEV, Lösante Hospital, Ankara, Turkey
3MSc Molecular Biologist, Tissue Typing and Immunology Laboratory LÖSEV, Lösante Hospital, Ankara, Turkey
4MD, Prof. of Pediatric Hematology, Manager of Tissue Typing and Immunology Laboratory LÖSEV, Lösante Hospital, Ankara, Turkey
5MD, Pediatric Hematologist, Founder of LÖSEV, LÖSEV, Lösante Hospital, Ankara, Turkey
6MD, Prof. of Pediatric Hematology, Board Member of Lösante Hospital LÖSEV, Lösante Hospital, Ankara, Turkey
*Corresponding Author: Pınar Akpınar Oktar, Dr. Biologist, Operational Director of Tissue Typing and Immunology Laboratory Lösante Hospital Kızılcaşar, 23 Nisan St. No:20, 06830 Gölbaşı/Ankara, Turkey.
| ReceivedDec 20, 2022 | RevisedJan 2, 2023 | AcceptedJan 5, 2023 | PublishedJan 28, 2023 |
Abstract
Objective: According to risk classifications, the transplant of healthy hematopoietic stem cells in the bone marrow is a priority treatment option for hematopoietic diseases. The effectiveness of hematopoietic stem cell transplantation after bone marrow transplantation is largely dependent on the capacity of the bone marrow microenvironment to accept transplanted cells. Detailed analysis of hematopoietic stem cell kinetics after transplantation is very important, as initial transplantation of hematopoietic stem cells affects prognosis. In this study, hematopoietic stem and progenitor cell percentage values were evaluated together with other transplantation-related parameters to determine the capacity of stem cell dynamic variables in the bone marrow.
Methods: Dynamic variables in hematopoietic stem cell fate in bone marrow transplantation include many parameters; hematopoietic stem cell transplantation rates in the apheresis product of 13 patients and cell counts per kilogram and engraftment times, CD34+ cell rates, CD34+ cell counts per kilogram, chimerism rates, Graft-versus-Host Disease grades, white blood cell counts, and post-transplant relapse status. In the apheresis products collected from the patients before transplantation, hematopoietic stem and progenitor cells were identified by the immunophenotypic barcode "CD34+, CD38-, CD45RA-, CD90+, CD49f+", and data analysis was performed with the BD FACSDivaTM software by studying on the BD FACSCanto II flow cytometer device.
Results: According to the results of the pearson correlation analysis (non-parametric correlations), a strong positive and significant relationship was found between the number of hematopoietic stem cell transplantation per kg and the percentage of hematopoietic stem cell transplantation of the patients (r=.733, p<.05). According to the results of the analysis, there was a moderately significant and positive relationship (r=.631, p<.05) between the percentage of hematopoietic stem cell transplantation and platelet engraftment time, while there was a strong inverse relationship between erythrocyte engraftment time (r=-.730, p<.01). A strong positive and significant relationship was found between the number of Hematopoietic stem cell transplantation cells per kilogram infused into the patients and the platelet engraftment times (r=.780, p<.05).
Conclusion: This study confirms that a mean cell dose of hematopoietic stem and progenitor cells ≥ 2.57 x 106/kg infused provides rapid short- and long-term platelet engraftment in pediatric patients undergoing autologous and allogeneic transplantation. This study is important in terms of revealing the possible relationship between pulling forward the platelet engraftment time and the number and percentage of Hematopoietic stem and progenitor cells in our transplanted patients. It is also important that a statistically significant inverse relationship was determined between HSPC values and mean erythrocyte engraftment times.
Keywords
Acute leukemia; Bone marrow transplantation; Hematopoietic stem and progenitor cell, Engraftment; Flow cytometry; Immunophenotypic barcode; Pediatrics
Introduction
Hematopoietic stem cell transplantation (HSCT) is a medical procedure that infuses stem cells after a short course of chemotherapy, radiotherapy, or both namely a conditioning regimen [1]. Bone marrow transplantation has become a curative therapy for an increasing number of malignant and non-malignant diseases [2]. HSCT was first performed by E. Donnell Thomas in 1957 as a new form of cancer therapy [3]. Although initial attempts were largely unsuccessful, the procedure has improved dramatically over the past decades [4]. Today, more than 50,000 HSCT procedures are performed annually for a variety of malignant and benign diseases worldwide [5]. In an HSCT procedure, a recipient's unhealthy natural bone marrow cells and immune system are replaced with grafted healthy stem cells and immune cells (grafts) after a brief chemotherapy and/or radiotherapy administration. The procedure can eliminate residual cancer by taking advantage of the graft-tumor effect. The primary goal of most transplants is to treat an underlying malignancy or hematological disorder [1]. Detailed analysis of hematopoietic stem cell (HSC) kinetics after transplantation is very important, as initial transplantation of HSCs affects prognosis. Although the number of autologous and allogeneic stem cell transplantations is increasing, relatively little information is available about recovery after transplantation. The adult bone marrow (BM) niche is a complex space in which the dynamic homeostatic and hematopoietic system is converged between different cellular and non-cellular factors. Signaling mechanisms triggered by cell-cell, cell-extracellular matrix, cell-cytokine interactions, and local microenvironment parameters play a role in the self-renewal and differentiation of stem cells and the control of the migration of hematopoietic stem and progenitor cells (HSPCs) [6]. It is also an important question to what extent the amounts given during bone marrow transplantation (BMT) of HSCTs, which have significant potential in many ways controlled by the dynamics of the BM niche, affect the transplantation. In their study, Carroll, et al., stated that the in vitro leukemic niche they created for understanding the molecular events responsible for the functional failure of HSCT may be useful for therapeutic evaluation [7]. In addition, medicinal products based on autologous HSCTs created using lentiviral and gammaretroviral vectors are now approved for clinical use [8]. In this study, to determine the optimal dose of HSCT for rapid and stable engraftment after HSCT, the relationship between engraftment times, which is one of the most important variables of the hematopoietic healing process, and HSCT cells infused during transplantation was analyzed.
Material and method
Peripheral blood progenitor cells (PBPC) were mobilized with granulocyte colony-stimulating factor (G-CSF) alone (10 μg/kg/day SC, Neupogen (R); Amgen, Thousand Oaks, California, USA) and a Spectra Optia Apheresis System-Terumo BCT (Colorado, Colorado, USA) and mononuclear cells including CD34+ cell contents were collected. Immunophenotypic analyses were performed on samples from apheresis products by flow cytometry. Local protocols and techniques routinely used at our center were used for instrument calibration, sample preparation, immunostaining, and data collection [9,10]. CD34+ cells were studied with the BD Stem Cell Enumeration kit (Cat No: 344563). HSPC’s markers; PerCpCy5.5-CD34 (Cat No: 347222), APCH7-CD38 (Cat No: 656646), APC-CD45RA (Cat No:550855), PE-CD49f (Cat No: 555736) and PeCy7-CD90 (Cat No: 561558), BV510/500-CD45 (Cat No: 655873) were determined by the expression of cell surface markers. Prepared cells were read at low speed on a BD FACS Canto II flow cytometer and the data analysis software program BD FACS Diva TM software was used for data analysis.
Engraftment is an important indicator for the evaluation of graft function in the early post-transplantation period, and the following criteria were evaluated and followed up:
a) Neutrophil engraftment: The first day when the fragmented neutrophil count is >500/mm³ for 3 consecutive days.
b) Platelet engraftment: The first day when the platelet count is >20.000/mm3 for 3 consecutive days without platelet support for 7 days.
c) Erythrocyte engraftment: The first day when the reticulocyte count is >60.000/mm³ for 3 consecutive days.
Measurements were made on the Sysmex XN-1000 SA-01 hemogram device to determine neutrophil, platelet, and erythrocyte engraftment times.
Result
In this study, 13 pediatric patients aged between 2 and 15 years underwent 18 HSCT procedures for acute lymphoblastic leukemia (ALL), acute myeloblastic leukemia (AML), lymphoma, and neuroblastoma diseases (able 1). After relapse, 2nd HSCT was performed in 1 of the patients and 3rd HSCTs were performed in 2 of the patients. Due to the recurrence of malignancies in 3 of the patients, 2nd and 3rd HSCTs were performed after relapse. To analyze the relationships between variables affecting HSCT and HSCT cell values, each transplant of 3 patients with more than one transplantation was considered a different transplantation procedure.
The cumulative incidence of chronic GvHD was determined as 38.8% in our 18 HSCT procedures that make up our case series. In our case series, the mean platelet engraftment time was 19 days (Figure 1). As observed in Table 1, it has been shown that there is a statistically significant and positive correlation between the percentage and number of HSCT (M=0.29, SE=0.16; M=2.57 × 106, SE=1.83 × 106) and the platelet engraftment time (M=19.25, SE=3.62).
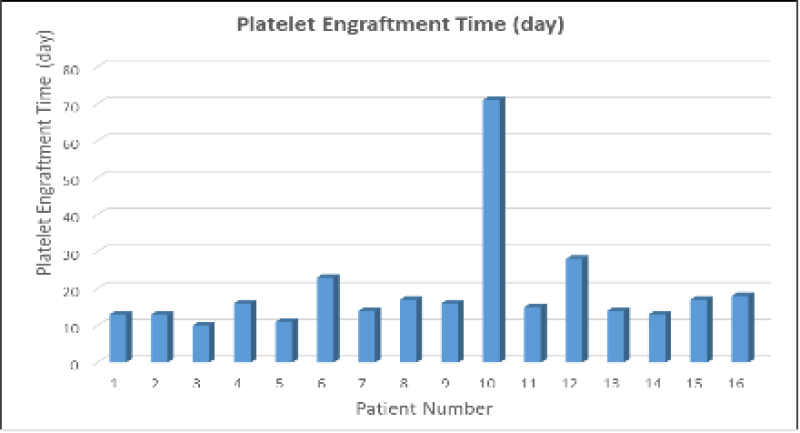
Figure 1: Platelet engraftment times in a series of 16 cases. In 16 case series with moderately significant and positive correlation (r=.631, p<.05) between HSPC percentage and platelet engraftment time, the mean platelet engraftment time was 19 days.
Count (n) | 13 | |
| Age (Year) | 2-15 | |
| Sex | Female | 6 |
| Male | 7 | |
Diagnosis | ALL | 7 |
| AML | 4 | |
| Lymphoma | 1 | |
| Neuroblastoma | 1 | |
HSCT* | Allogeneic | 15 |
| Autologous | 3 | |
| BM* | 8 | |
| Stem Cell Source | Peripheral | 10 |
Engraftment Time (number of patients) | Neutrophil | 18 |
| Platelet | 16 | |
| Erythrocyte | 16 | |
| Grade I-II GvHD (in allo-tx) | Yes | 6 |
| Grade III-IV GvHD (in allo-tx) | Yes | 1 |
Post HSCT | CC** | 15 |
| Relapse | 7 | |
| Ex | 2 |
Table 1: The demographic, clinical, and laboratory data of the patients. *A total of 18 HSCT procedures were evaluated for 13 patients; **CC: complete chimerism; After relapse, graft 2. HSCT of one patient and graft 3. HSCT of two patients; GVHD: Graft-versus-host Disease; HSCT: Hematopoietic Stem Cell Transplantation; BM: Bone Marrow.
In this study, dynamic variables in HSC fate in BMT in 13 pediatric patients included HSPC counts (c/kg) (calculated according to total product volume), HSPC percentage values (%), and engraftment (neutrophil, platelet, erythrocyte) times, CD34+ cell counts. Many parameters were evaluated, including (c/kg) (calculated by total product volume) CD34+ cell percentage values (%) (Table 2). In pediatric patients undergoing autologous transplantation, the CD34+ cell dose is planned to be ≥ 2.0 × 106/kg, and ≥ 5 × 106/kg in allogeneic transplants.
| Variable | M | SE | Platelet Engraftment Time (day) (r) | Erythrocyte Engraftment Time (day) (r) | Neutrophil Engraftment Time (day) (r) |
| HSPC% (n=14) | 0.29 | 0.16 | 0.631* | -0.730** | |
| HSPC cells/kg (n=14) | 2.57 × 106 | 1.83 × 106 | 0.780* | -0.419 | -0.294 |
| CD34+ cell% (n=14) | 2.99 | 1.93 | 0.189 | -0.17 | 0.252 |
| CD34+ cells/kg (n=14) | 8.15 × 106 | 1.83 × 106 | -0.745* | 0.05 | 0.232 |
| Neutrophil Engraftment Time (day) (n=18) | 17.94 | 1.01 | -0.244 | 0.483 | - |
| Platelet Engraftment Time (day) (n=16) | 19.25 | 3.62 | - | -0.335 | -0.244 |
| Erythrocyte Engraftment Time (day) (n=16) | 23.06 | 1.53 | -0.335 | - | 0.483 |
Table 2: Descriptive statistic and correlations for study variables. *p<.05; **p<.01; HSPC: Hematopoietic stem cell transplantation; WBC: White blood cell; M: Mean; SE: Standard error; r=Correlation coefficient.
According to the results of the Pearson correlation analysis (Nonparametric Correlations), a strong positive and significant relationship was found between the HSPC number per kg (M=2.57 × 106, SE=1.83 × 106) and the HSPC percentage values (M=0.29, SE=0.16) of the patients (r=.733, p<.05).
According to the results of the analysis, there was a moderately significant and positive relationship (r=.631, p<.05) between HSPC percentage and platelet engraftment time (M=19.25, SE=3.62), while erythrocyte engraftment time (M=23.06, SE=1.53) and the existence of a strong inverse relationship (r=-.730, p<.01).
In this study, the mean erythrocyte engraftment time was determined to be 23 days in a series of 16 cases (Figure 2).
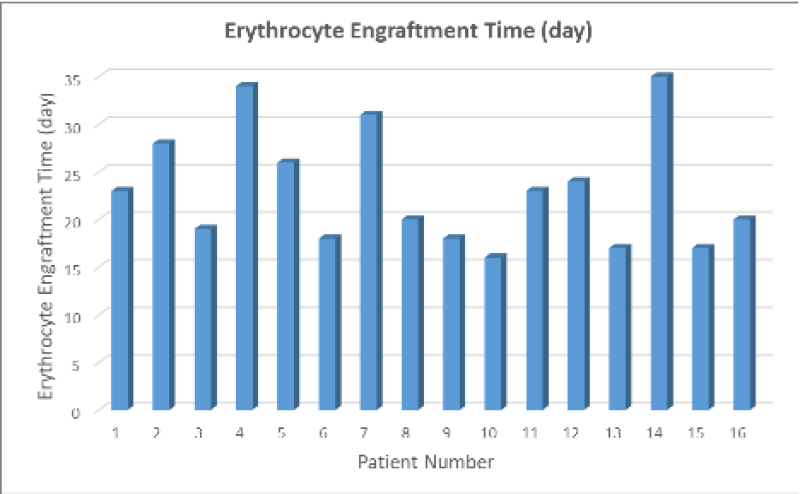
Figure 2: Erythrocyte engraftment times in a series of 16 cases. A strong inverse relationship was determined between HSPC percentage values and erythrocyte engraftment times (r=-.730, p<.01). The mean erythrocyte engraftment time was 23 days in a series of 16 cases.
The median CD34+ cell dose of all patients in allogeneic transplants in our study was 5.8 × 106/kg (range 1.5 to 27.2). The median platelet engraftment time of the patients was 15.5 (range 11 to 71) days, and the median erythrocyte engraftment time was 21.5 (range 16 to 35) days.
It was determined that there was a significant, inverse, and strong relationship between platelet engraftment time and CD34+ cell count per kilogram (M=8.15 × 106, SE=1.83 × 106) (r=.745, p<.05).
A strong positive and significant correlation was found between the number of HSPC per kilogram infused into patients (M=2.57 × 106, SE=1,83 × 106) and platelet engraftment times (r=.780, p<.05). No correlation was noted between neutrophil engraftment time, WBC, and numbers and percentages of HSPC infused per kilogram.
Discussion and Conclusion
This study confirms that G-CSF-mobilized PBPC provides rapid short-term and long-term platelet engraftment in pediatric patients undergoing autologous and allogeneic transplantation if the HSPC dose is infused on a mean of ≥ 2.57 × 106/kg. Since this dose of HSPC appears to have clinical and potential economic implications, it may be considered the optimal dose for apheresis. It is useful to determine the average value of the optimum HSPC dose by increasing the number of patients to answer the question of how much the amounts of HSPCs controlled by the dynamics of the BM niche affect the transplantation.
Ringden O, et al., the mean time to platelet engraftment was reported to be 23 days in the PBSC transplant patient group [11]. In the study, this period is 19 days. The study is important in terms of revealing the possible relationship between the shortening of platelet engraftment time and the number and percentage of HSPC in transplant patients.
It is also important that a statistically significant inverse relationship was determined between HSPC values and mean erythrocyte engraftment times. It would be beneficial to examine the relationship between these factors in more detail and in a high number of patient populations.
Kulkarni U, et al., showed in their study that the median time to platelet engraftment was 17 days (range, 10 to 44) in patient groups whose median CD34 cell dose was 4.87 × 106/kg [12]. In our study, the median CD34+ cell dose was 5.8 × 106/kg (range 1.5 to 27.2) while the median platelet engraftment time was 15.5 (range 11 to 71). A study shows, the pulling forward of the median platelet engraftment time may also be associated with an increase in the dose of median CD34+ cells and HSPC. It may positively affect platelet engraftment, especially in the allogeneic transplant process.
The data obtained can provide a wide range of predictions for evaluating recovery before and after transplantation.
References
1. Bazinet A, Popradi G. A General Practitioner’s Guide to Hematopoietic Stem-cell Transplantation. Curr Oncol. 2019;26(3):187-91. PubMed | CrossRef
2. Frederick R, Appelbaum MD. Hematopoietic Cell Transplantation. N Engl J Med. 2007;2:668-73. PubMed | CrossRef
3. Thomas ED, Lochte Jr HL, Lu WC, Ferrebee JW. Intravenous Infusion of Bone Marrow in Patients Receiving Radiation and chemotherapy. N Engl J Med. 1957;257(11):491-6. PubMed | CrossRef
4. World Health Organization. Haematopoietic Stem Cell Transplantation HSCtx [Web page] Geneva, Switzerland: WHO. 2018.
5. Costa MH, De Soure AM, Cabral JM, Ferreira FC, Da Silva CL. Hematopoietic Niche-exploring Biomimetic Cues to Improve the Functionality of Hematopoietic Stem/Progenitor Cells. Biotechnol J. 2018;13(2):1700088. PubMed | CrossRef
6. Carroll D, ST Clair DK. Hematopoietic Stem Cells: Normal Versus Malignant. Antioxid Redox Signal. 2018;29(16):1612-32. PubMed | CrossRef
7. Van Dongen JJ, Lhermitte L, Böttcher S, Almeida J, Van Der Velden VH, Flores-Montero J, et al. Euro Flow Antibody Panels for Standardized N-dimensional Flow Cytometric Immunophenotyping of Normal, Reactive and Malignant Leukocytes. Leukemia. 2012;26(9):1908-75. PubMed | CrossRef
8. Ferrari G, Thrasher AJ, Aiuti A. Gene Therapy Using Haematopoietic Stem and Progenitor Cells. Nat Rev Genet. 2021;22(4):216-34. PubMed | CrossRef
9. Kalina T, Flores Montero J, Van Der Velden VH, Martin Ayuso M, Böttcher S, Ritgen M, et al. Euro Flow Standardization of Flow Cytometer Instrument Settings and Immunophenotyping Protocols. Leukemia. 2012;26(9):1986-2010. PubMed | CrossRef
10. Ringden O, Remberger M, Runde V, Bornhäuser M, Blau IW, Basara N, et al. Faster Engraftment of Neutrophils and Platelets with Peripheral Blood Stem Cells from Unrelated Donors: A Comparison with Marrow Transplantation. Bone Marrow Transplant. 2000;25(2):6-8. PubMed | CrossRef
11. Kulkarni U, Devasia AJ, Korula A, Fouzia NA, Nisham PN, Samoon YJ, et al. Use of Non-cryopreserved Peripheral Blood Stem Cells is Associated with Adequate Engraftment in Patients with Multiple Myeloma Undergoing an Autologous Transplant. Biol Blood Marrow Transplant. 2018;24(12):31-5. PubMed | CrossRef
12. Horowitz MM. Uses and growth of hematopoietic cell transplantation. Thomas’ hematopoietic cell transplantation: Stem cell transplantation. 2015;1:8-17.
Pınar Akpınar Oktar1*, Merve Güneş2, Rumeysa Kılıç3, Türkan Patıroğlu4, Üstün Ezer5 and Ahmet Emin Kürekçi6
1Dr. Biologist, Operational Director of Tissue Typing and Immunology Laboratory Lösante Hospital Kızılcaşar, 23 Nisan St. No:20, 06830 Gölbaşı/Ankara, Turkey
2MSc. Bioengineer, Tissue Typing and Immunology Laboratory LÖSEV, Lösante Hospital, Ankara, Turkey
3MSc Molecular Biologist, Tissue Typing and Immunology Laboratory LÖSEV, Lösante Hospital, Ankara, Turkey
4MD, Prof. of Pediatric Hematology, Manager of Tissue Typing and Immunology Laboratory LÖSEV, Lösante Hospital, Ankara, Turkey
5MD, Pediatric Hematologist, Founder of LÖSEV, LÖSEV, Lösante Hospital, Ankara, Turkey
6MD, Prof. of Pediatric Hematology, Board Member of Lösante Hospital LÖSEV, Lösante Hospital, Ankara, Turkey
*Corresponding Author: Pınar Akpınar Oktar, Dr. Biologist, Operational Director of Tissue Typing and Immunology Laboratory Lösante Hospital Kızılcaşar, 23 Nisan St. No:20, 06830 Gölbaşı/Ankara, Turkey.
Copyright© 2023 by Akpınar Oktar P, et al. All rights reserved. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Akpınar Oktar P, Güneş M, Kılıç R, Patıroğlu T, Ezer U, Kürekçi AE. Evaluation of Transplanted Stem Cell Dynamic Variables in the Bone Marrow of Children with Malignancies. J Biomed Allied Res. 2023;5(1):1-9. DOI: https://doi.org/10.37191/Mapsci-2582-4937-5(1)-030
