JOURNAL OF REGENERATIVE BIOLOGY AND MEDICINE
Anti-inflammatory and Antifibrotic Effects of Plant Regulatory Peptides in Patients with Steatohepatitis and Cirrhosis: Opportunities of Primary Liver Cancer Prevention
| ReceivedOct 1, 2019 | RevisedOct 4, 2019 | AcceptedOct 15, 2019 | PublishedOct 22, 2019 |
Anatolyy Pechinka¹, Levan Kakliani², Zurab Gogitidze³, Sergii Konovalenko4*and Iryna Miroshnychenko5
1Department of Infectious Diseases, Shupyk National Medical Academy of Postgraduate Education, Kiev, Ukraine
2Department of Internal Medicine, University Hospital of Tbilisi, Georgia
3Regul Medical Information Center, Tbilisi, Georgia
4RE Kavetsky Institute of Experimental Pathology, Oncology and Radiobiology, Kyiv, Ukraine
5Kyiv City Center for Radiation Protection of Kyiv Population from the Consequences of the Chornobyl Catastrophe, Ukraine
*Corresponding Author: Sergii Konovalenko, RE Kavetsky Institute of Experimental Pathology, Oncology and Radiobiology, Kyiv, Ukraine, Tel: +38 (067) 377 81 33.
Received Date:10-01-2019; Accepted Date:10-15-2019; Published Date:10-22-2019
Copyright© 2019 by Konovalenko S, et al. All rights reserved. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The pathogenesis of steatohepatitis, which develops on the background of non-alcoholic fatty liver disease and may be the cause of liver cirrhosis, is considered in the article. Also, the paper focuses on the prospects of using peptide drugs for the treatment of liver cirrhosis. The study found that the preparation of plant regulatory peptides GA-40 has pronounced hepatoprotective, anti-inflammatory and anti-fibrotic effects, which is confirmed by the elastometry data of the shear wave. The results of this work indicate that the use of the drug GA-40 was accompanied not only by the normalization of the inflammatory panel of the liver and triglycerides, but also by a likely decrease in the level of glypican-3 - the main factor that causes the development of primary liver cancer. Gene protector and immunocorrector GA-40, harmonizing metabolic processes in the liver, as well as suppressing inflammation and preventing excessive output of hepatocytes in apoptosis, has a positive effect on the prognosis of the disease. Reduced expression of glypican-3 in patients in the study group indicates that GA-40 is able to control the level of pro-oncogenic trigger proteins, thus reducing the risk of primary liver cancer.
Keywords
Steatohepatitis; Cirrhosis; Glypican-3; Ga-40; Hepatocellular Carcinoma
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a disease that results from excessive fat accumulation (mainly triglycerides) in the form of steatosis in the liver in people who do not consume alcohol in amounts capable of causing liver damage [1,2]. NAFLD and non-alcoholic steatohepatitis (NASH) are more common in women aged 40-60 years, but there are reports of this type of pathology in younger patients. NAFLD is a type of liver steatosis or fatty hepatosis that occurs in people who do not abuse alcohol and is most commonly associated with insulin resistance and metabolic syndrome. The term "NAFLD" has several synonyms: non-alcoholic liver steatosis, fatty liver, fatty infiltration of hepatocytes. [3,5,8].
One of the most characteristic phenomena in this disease is the accumulation of fat in the functioning tissue of the liver. In the future, it leads to the replacement of this tissue with adipose tissue, which eventually causes the development of chronic inflammation. Isolated liver steatosis is a relatively benign condition with minimal risk of progression to more severe liver disease [4,6,7,9]. The development of inflammatory processes on the background of fatty dystrophy leads to damage hepatocytes and the progress of non-alcoholic, or metabolic, steatohepatitis, which is one of the stages of NAFLD, and steatohepatitis gradually leads to liver cirrhosis [10,11]. This mechanism also works with viral, toxic (alcohol) lesions as an additional damaging factor. Non-alcoholic fatty liver disease occupies one of the leading positions among diffuse liver diseases. The relevance of the study of NAFLD is due to both the prevalence of the disease and the risk of developing cirrhosis and hepatocellular carcinoma (HCC) (Figure 1). The proportion of patients with NAFLD in the adult population ranges from 6.3% to 33% with a median of 20% in the global population. In some countries, the incidence is 46% [12]. The progression of the disease also increases the risk of liver cirrhosis and liver failure. Studies have shown that a quarter (27%) of patients with NAFLD develop fibrosis within 9 years, and one in five (19%) have cirrhosis of varying degrees of severity [13,14]. It is established that the stage of fibrosis, and not the severity of inflammation, is the prognostic factor that determines the subsequent fate of patients and the likelihood of complications. In this regard, it is important to look for predictors of progression of liver fibrosis to individualize therapy and follow-up of the patient. At the present stage, it seems interesting to study gene polymorphisms in the development or progression of diseases.
The G-allele of the PNPLA3 gene is positively correlated with the triglyceride content of liver tissue, and its loss of activity may be directly related to inflammation of the liver tissue. Concerning the association of PNPLA3 with the development and progression of liver fibrosis in histologically confirmed NAFLD, regardless of obesity, diabetes mellitus and steatosis, convincing data have also been presented [16]. The participation of this gene in liver cirrhosis formation and transformation in HCC has been proved [17], regardless of external factors (obesity, alcohol consumption). The polymorphism of the PNPLA3/148M gene is today an independent genetic factor for the development and progression of NAFLD and non-alcoholic steatohepatitis.
Figure 1: Scheme of progression of non-alcoholic fatty liver disease.
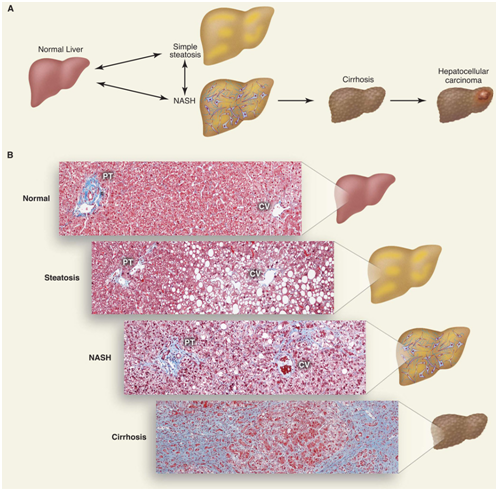
A. Accumulation of lipid (triglyceride) droplets inside hepatocytes causes steatosis. Steatosis associated with inflammation, cell death and fibrosis progresses and non-alcoholic steatohepatitis develops, which can turn into cirrhosis. People with cirrhosis have an increased risk of developing hepatocellular carcinoma.
B. Histological sections showing the normal state of the liver, steatosis, steatohepatitis and cirrhosis. Collagen fibers are colored in blue by the Masson method. A portal triad (PT) consisting of a hepatic artery, a portal vein, a bile duct and a central vein (CV) is shown [15].
Glypican-3 (GPC3), an oncofetal proteoglycan, attaches to the cell membrane, usually found in the liver of the fetus, but not in the healthy liver of the adult. However, in patients with HCC GPC3 is overexpressed and its expression predicts poor prognosis. Studies have shown that GPC3 functions in the progression of HCC by binding to molecules such as Wnt signaling proteins and growth factors [18-20].
According to D. Baumhoer et al. [21], positive expression of glypican-3 is also observed in individual high-grade dysplastic nodules, indicating their carcinogenic orientation. Elevation of glypican-3 is also considered as a prognostic factor for HCC in patients with cirrhosis. Glypican can enhance mRNA expression of HCC-related genes and participate in the neoplastic process in the cirrhotic liver. Therefore, GPC3 can serve as a precursor biomarker in liver cirrhosis [22,23]. Moreover, GPC3 was used as a target for molecular imaging and therapeutic intervention in HCC [24]. Magnetic resonance imaging focused on GPC3, positron emission tomography and near-infrared tomography for early detection of HCC have been investigated, various GPC3 immunotherapeutic protocols have been developed, including the use of humanized anti-cytotoxic DNA/cytotoxic treatments, immunotoxin therapy, and genetic therapy [25,26]. GA-40 is the first gene protector and immunocorrector based on plant regulatory peptides to be used in clinical practice. It is known that GA-40 peptides, due to their relatively small size (15-98 kDa), can affect not only membrane receptors but also penetrate cells through membrane pores. This enables them to interact with intracellular structures: regulatory peptides and genes, ligands and enzymes. Studies have also demonstrated that GA-40 peptides are intracellularly capable of altering the active potential of participants in the regulatory cascades: mRNA-associated proteins, caspases, protein kinases, prointerleukins, interleukins, and the like. The history of the discovery and study of the immunotropic and gene-protective properties of GA-40 is set out in Professor George Alexidze's monograph “GA-40. New Immunotherapy and Anticancer Drug†which was released in 2014 in the USA- Lambert Academic Publishing (Feb 12, 2014).
The uniqueness of GA-40 is that plant peptides under conditions of reduced antitumor immunity due to aggressive tumor activity or after antitumor chemotherapy restore immune surveillance and promote apoptosis of cancer cells [27]. In opposite conditions, that is, in conditions of hyperproduction of immunocompetent molecules in inflammatory processes, in particular in the liver and kidneys (in hepatitis, cirrhosis, nephritis), plant peptides are able to reduce the intensity of peptide-peptide interactions and prevent undesirable damage to healthy Ñells [28]. This paradox can be explained by the evolutionarily formed properties of regulatory peptides-self-assembling and the natural intention for harmonization.
Aim of Research
In order to study the hepatoprotective, anti-inflammatory and antifibrotic action of GA-40, we formed two groups of patients diagnosed with steatohepatitis, liver cirrhosis.
Materials and Methods
Based on the Department of Therapy of the University Clinic of Tbilisi (Georgia), from 2016 to 2019 experience in the use of GA-40 was obtained in 34 patients (25 women and 9 men aged 44 to 65 years) with steatohepatitis [23] and cirrhosis of the liver [11]. To study the hepatoprotective, anti-inflammatory and anti-fibrotic properties of GA-40, a control group of patients-C (N=34) was recruited.
The study group received the following treatment: ademetionine 400 mg 2 times a day tablets orally, verospiron capsules 50 mg 2 times a day, triamterene with hydrochlorothiazide (25 mg+12.5 mg tablets) orally and GA-40 on a pulse schedule-400 μg of solution of regulatory peptides per day injected intramuscularly for 28 days, total of 6 courses for 28 days with breaks in 1 week. The control group received therapy according to the scheme: ademetionin 400 mg 2 times a day tablets orally, verospiron capsules 50 mg 2 times a day, triamterene with hydrochlorothiazide (tablets 25 mg+12.5 mg) orally. We monitored ALT, AST, GGT, C-reactive peptide, and triglyceride levels for treatment at 35, 75, 105, 140, 175, and 210 days of follow-up. Such distribution of control points is conditioned by the scheme of therapy in the study group: 28 days is the introduction of GA-40, then 7 days of break-and control for 35 days, then another 28 days of therapy, 7 days of break-and control for 70 days, etc. As a marker of the risk of primary liver cancer, we used the expression level of glypican-3 (GPC-3). The level of fibrosis was controlled by ultrasound-shear elastometry. The study group had 11 patients with liver cirrhosis in stage F4 on a METAVIR scale with different levels of fibrosis. Indicators were monitored before treatment and after treatment.
Results and Discussion
The study groups were comparable in the pre-treatment phase (p> 0.05). Clinical observations show that in the study group in the first month of therapy there was a tendency to normalize ALT, AST and other biochemical indicators of liver inflammation. Gradually decreased serum triglyceride levels (Table 1). Similar dynamics of biochemical parameters were recorded in the control group, especially in the first 2 months of observation, but on the 105th and 175th day, the AST marker level increased slightly-to an average of 54.3 IU/l and 49.5 IU/l, respectively. , which formed a statistically significant difference compared to the study group (p<0.05). A statistically significant difference between the ALT groups is formed from 105 days of observation, and the AST level from 35 days is maintained until the end of the study (p<0.05). With quite positive dynamics of decrease in inflammatory marker GGT in the control group, on day 210, there was a slight increase in it from an average of 21.8 to 34.7 IU/l (p<0.05 when compared between groups). Also, in the control group, expression of the C-reactive peptide after a marked decrease from the level of 9.1 IU/ml to 4.9 IU/ml increased again at the sixth control point to 7.2 IU/ml (p<0.05) (Table 2) It should be noted that there were no such increases in the study group and the levels of AST, ALT, GGT and C-reactive peptide remained within the normal range throughout the observation period from the second control point (Figure 2).
Table 1: Biochemical parameters in dynamics in patients of study group.
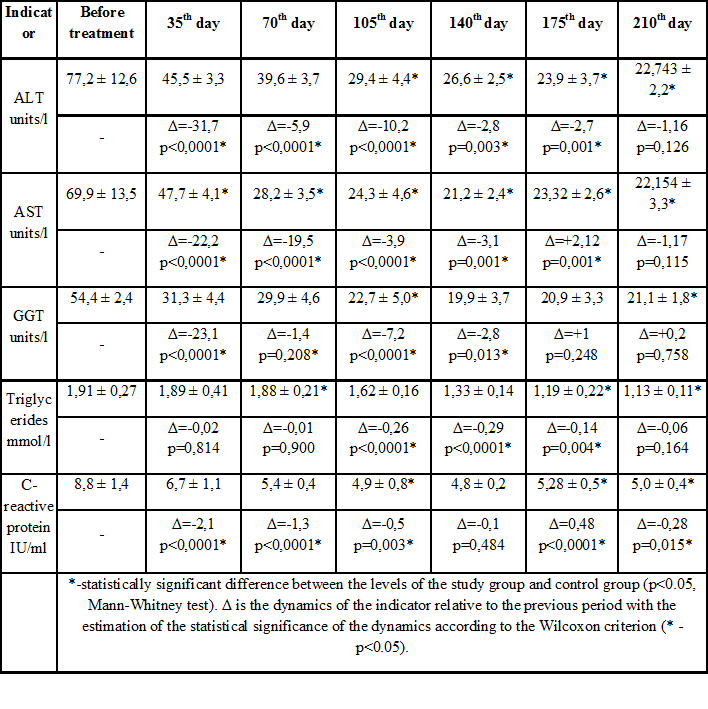
Table 2: Biochemical parameters in dynamics in patients of control group.
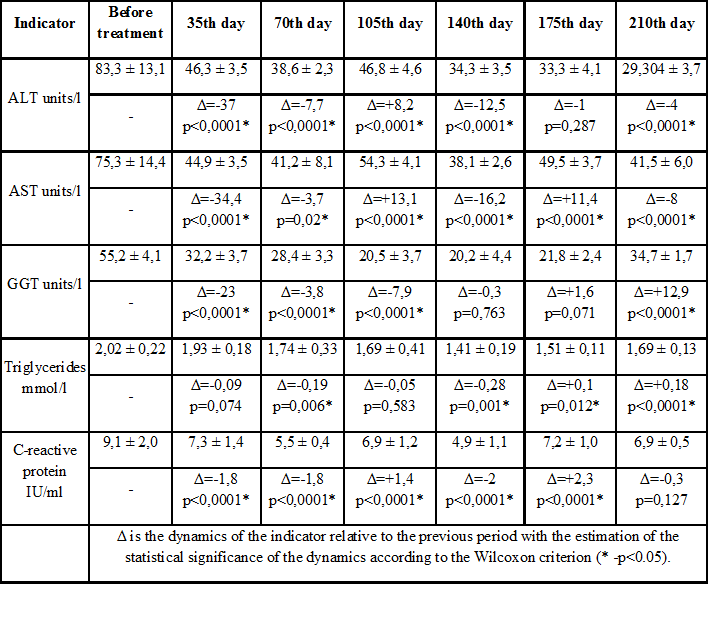
Figure 2: Level of glypican-3 in patients during treatment.
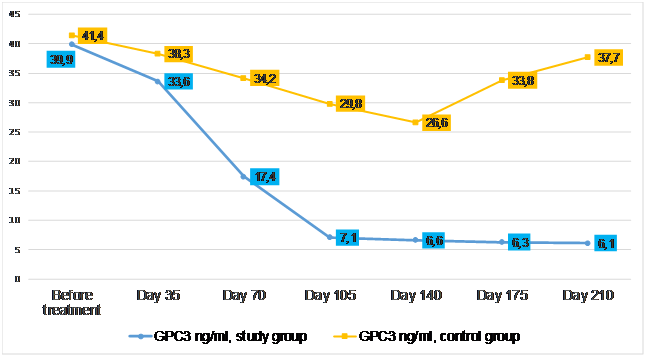
Equally interesting was the assessment of glypican-3 (GPC3) protein expression in patients in the study and control groups. In the first five weeks after the start of the treatment protocol, there was a slight decrease in serum GPC3 concentration from 39.9-41.4 to 33.6-38.3 ng/ml in both observation groups. However, at the third point of control, on the 70th day from the start of pulse therapy, the average expression of GPC3 in patients in the study group showed a sharp decrease to 17.4 ng/ml (p<0.05). This trend persisted-on day 105 of the treatment course, the level of GPC3 in the patients in the study group dropped to 7.1 ng/ml and remained relatively low throughout the observation period. At the same time, GPC3 expression reached the level of 26.6 ng/ml on day 140 of the treatment protocol in patients of the control group, and after 5 weeks it increased significantly again-up to 33.8 ng/ml. At the final reference point, GPC3 activity increased again to 37.7 ng/ml, almost returning to baseline. A statistically significant difference between the groups (p<0.05) begins to form after 35 days of observation. All 11 patients with cirrhosis in the study group had a statistically significant decrease in liver fibrosis. In 3 of them, to the F3 level on the METAVIR scale, and in the rest-to the initial F4 level: from 35.3 kPa on average, there was a decrease to 12.7 kPa.It can be reasonably assumed that the gene protector and immunocorrector GA-40, by harmonizing metabolic processes in the liver, as well as suppressing inflammation and preventing excessive hepatocytes exit into apoptosis, have a positive effect on the prognosis of the disease. Reduced expression of glypican-3 in patients in the study group indicates that GA-40 with a high probability is capable of controlling the level of pro-oncogenic trigger proteins, thus reducing the risk of primary liver cancer.
Conclusions
The results of this work indicate that the use of the drug GA-40 was accompanied not only by the normalization of the inflammatory panel of the liver and triglycerides, but also a likely decrease in the level of glypican-3. The results obtained confirm that the preparation of plant regulatory peptides GA-40 has pronounced hepatoprotective, anti-inflammatory and anti-fibrotic effects, which is confirmed by the elastometry data of the shear wave. Against the background of complex treatment, not only was the gradual regression of the clinical symptoms of the disease, but there was a significant improvement in the general condition and quality of life of patients. At this stage, the inclusion of GA-40 in the treatment regimens of steatohepatitis and cirrhosis of the liver is quite reasonable, especially since this drug is able to significantly reduce the risk of hepatocellular carcinoma and significantly reduce the degree of liver fibrosis. Such properties make it possible to consider GA-40 as a potential drug for delaying or even avoiding the terminal stage of liver cirrhosis, in which indications for transplantation arise. The study of the effectiveness of the gene protector and immunocorrector GA-40 in liver pathology should certainly be continued.
References
1. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005-23.
2. Bae JC, Rhee EJ, Lee WY, Park SE, Park CY, Oh KW, et al. Combined effect of nonalcoholic fatty liver disease and impaired fasting glucose on the development of type 2 diabetes: a 4-year retrospective longitudinal study. Diabetes Care. 2011;34(3):727-9.
3. Bril F, Barb D, Portillo-Sanchez P, Biernacki D, Lomonaco R, Suman A, et al. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology. 2017;65(4):1132-44
4. Dongiovanni P, Stender S, Pietrelli A, Mancina RM, Cespiati A, Petta S, et al. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J Intern Med. 2018;283(4):356-70.
5. Fricker ZP, Pedley A, Massaro JM, Vasan RS, Hoffmann U, Benjamin EJ, et al. Liver fat is associated with markers of inflammation and oxidative stress in analysis of data from the framingham heart study. Clin. GastroenterolHepatol. 2019;17(6):1157-64.e4.
6. Jelenik T, Kaul K, Séquaris G, Flögel U, Phielix E, Kotzka J,et al. Mechanisms of insulin resistance in primary and secondary nonalcoholic fatty liver. Diabetes. 2017;66(8):2241-53.
7. Kim D1, Kim WR, Kim HJ, Therneau TM. Association between non-invasive fibrosis markers and mortality among adults with non-alcoholic fatty liver disease in the United States. Hepatology. 2013;57(4):1357-65.
8. Kwok R, Choi KC, Wong GL, Zhang Y, Chan HL, Luk AO, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65(8),1359-68.
9. Aleksandrova K, Boeing H, Nöthlings U, Jenab M, Fedirko V, Kaaks R, et al. (2014). Inflammatory and metabolic biomarkers and risk of liver and biliary tract cancer. Hepatology. 2014;60,858-71.
10. Wilfred de Alwis NM, Day CP. Genetics of alcoholic and nonalcoholic fatty liver disease. Semin. Liver Dis. 2007;27(1):44-54.
11. Kazankov K, Jørgensen SMD, Thomsen KL, Møller HJ, Vilstrup H, George J, et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. GastroenterolHepatol. 2019;16(3):145-59.
12. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) and European Association for the Study of Obesity (EASO). Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388-402.
13. Mary E. Rinella Nonalcoholic Fatty Liver Disease: A Systematic Review JAMA. 2015;313(22):2263-73.
14. Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, LoombaR.Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. ClinGastroenterolHepatol. 2015;13(4):643-54
15. Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332(6037):1519-23.
16. Chen LZ, Xin YN, Geng N, Jiang M, Zhang DD, Xuan SY. PNPLA3 I148M variant in nonalcoholic fatty liver disease: Demographic and ethnic characteristics and the role of the variant in nonalcoholic fatty liver fibrosis. World J Gastroenterol. 2015;21(3):794-802.
17. Dongiovanni P, Donati B, Fares R, Lombardi R, Mancina RM, Romeo S, et al. PNPLA3 I148M polymorphism and progressive liver disease. World J Gastroenterol. 2013;19(41): 6969-78.
18. Zhou F, Shang W, Yu X, Tian J.Glypican-3: A promising biomarker for hepatocellular carcinoma diagnosis and treatment. Med Res Rev. 2018;38(2):741-67.
19. Zhou R, Pan QJ, Wu K, Li R. Distribution and characterizationof glypican-3 in hepatocelluar carcinoma. Int J ClinExpPathol 2016;9:8631-3.
20. Haruyama Y, Kataoka H. Glypican-3 is a prognostic factor and an immunotherapeutic target in hepatocellular carcinoma.World J Gastroenterol. 20167;22(1):275-83.
21. Baumhoer D, Tornillo L, Stadlmann S, Roncalli M, Diamantis EK, Terracciano LM. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: a tissue microarray analysis of 4,387 tissue samples.Am J ClinPathol. 2008;129(6):899-906.
22. Liu X, Wang SK, Zhang K, Zhang H, Pan Q, Liu Z, et al. Expression of glypican 3 enriches hepatocellular carcinoma development-related genes and associates with carcinogenesis in cirrhotic livers.Carcinogenesis. 2015 ;36(2):232-42.
23. Tangkijvanich P, Chanmee T, Komtong S, Mahachai V, Wisedopas N, Pothacharoen P, et al. Diagnostic role of serum glypican-3 in differentiating hepatocellular carcinoma from non-malignant chronic liver disease and other liver cancers. J GastroenterolHepatol. 2010;25(1):129-37.
24. Ivanova II. May Glypican-3 be a novel biomarker and potential therapeutic target in hepatocellular cancer? J of IMAB. 2018;24(1):1936-40.
25. Abou-Alfa GK, Yen CJ, Hsu CH, O’Donoghue J, Beylergil V, Ruan S, et al. Phase Ib study of codrituzumab in combination with sorafenib in patients with non-curable advanced hepatocellular carcinoma (HCC). Cancer ChemotherPharmacol 2017;79 421-9.
26. Nguyen TB, Roncalli M, Di Tommaso L, Kakar S. Combineduse of heat-shock protein 70 and glutamine synthetase is useful in the distinction of typical hepatocellular adenomafrom atypical hepatocellular neoplasms and well-differentiated hepatocellular carcinoma. Mod Pathol 2016;29:283-92.
27. KA Galakhin, VF Konovalenko, TV Gordienko. New neoadjuvant (GA-40) in complex treatment of skin melanoma patient. Pathologia. 2008;5(1):28-33.
28. Rohovyy YE, Pechinka AM., Gogitidze ZD, Konovalenko SV. Counteraction in toxication with heavymetals: the unique nephro protective effects o fplantpeptides. Practicing doctor. 2019;8(3).
