JOURNAL OF CARDIOLOGY AND CARDIOVASCULAR RESEARCH
On this page
Direct Stenting in ST-elevation Myocardial Infarction: Comprehensive Review and Implications for Primary PCI Guidelines Inclusion
Rohit Mody1*, Abha Bajaj Nee Sheth2, Debabrata Dash3, Bhavya Mody4, Inderjeet Singh Monga5, Lakshay Rastogi6, Amit Munjal7, Bankey Bihari8 and Rahul Singla9
1MD, DM, Department of Cardiology, Mody Harvard Cardiac Institute and Research Centre, Krishna Super Specialty Hospital, Bathinda, Punjab, India
2MBBS, MS, Department of Anatomy, Doctor Harvansh Singh Judge Institute of Dental Sciences and Hospital, Panjab University, Chandigarh, India
3MD, DM, FICC, FAPSC, FCCP, FSCAI, Department of Cardiology, Aster Hospital, Mankhool, Dubai, Al Quasis, UAE
4MBBS, Department of Medicine, Kasturba Medical College, Manipal, Karnataka, India
5MBBS, DNB, DM, FSCAI, Department of Cardiology, Command Hospital Chandimandir, Panchkula, Haryana, India
6MBBS, Department of Medicine, Kasturba Medical College, Manipal, Karnataka, India
7MBBS, MD, DMB, Department of Cardiology, Doctor Asha Memorial Multispecialty Hospital, Fatehabad, Haryana, India
8MBBS, MD, Department of Medicine, Government Medical College and Rajindra Hospital, Patiala, Punjab, India
9MBBS, USMLE2, Extern, Internal Medicine, MedStar Franklin Square Medical Centre, Baltimore, USA
*Corresponding Author: Rohit Mody, MD, DM, Department of Cardiology, Mody Harvard Cardiac Institute and Research Centre, Krishna Super Specialty Hospital, Bathinda, Punjab, India.
| ReceivedMar 1, 2024 | RevisedMar 31, 2024 | AcceptedApr 14, 2024 | PublishedApr 28, 2024 |
Abstract
In the last two decades, primary percutaneous coronary intervention (PPCI) became the initial choice of management of patients who present as acute ST-segment elevation myocardial infarction (STEMI).
It is majorly used to achieve restoration of epicardial flow at earliest, to reduce the severity of myocardial injury. In spite of various advancements in percutaneous coronary intervention (PCI), subsequent no-reflow phenomenon and distal embolization are certain complications. Direct stenting (DS) without balloon pre-treatment is preferred therapeutic strategy which is associated with various advantages over conventional stenting (CS) like reduced no-reflow episodes, better angiographic outcomes, shorter time of procedure and fluoroscopy times and lower procedural cost.
However, long-term clinical outcomes of DS as compared to CS needs to be explored. This review article highlights various meta-analysis and prospective randomized studies between DS and CS in patients undergoing PCI presenting with STEMI. The authors are also describing retrospective observational study of DS in STEMI which was observed.
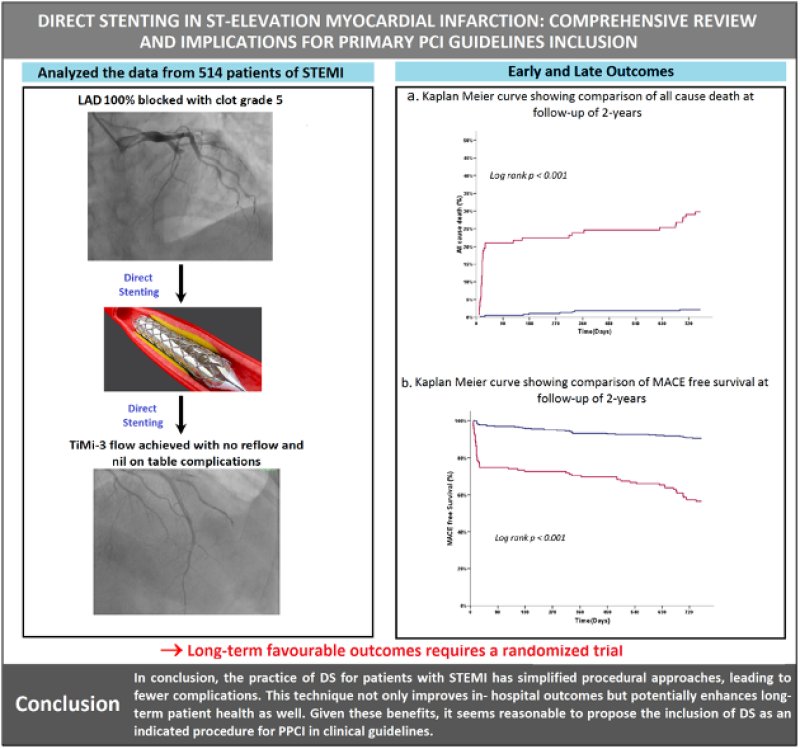
Figure 1: Direct stenting in STEMI and observational outcomes. Procedure is simplified by direct or direct like stenting with no-reflow phenomenon, no on-table complications and TIMI of grade-3 flow. No reflow phenomenon and nil on-table complications with TIMI flow of grade-3 was achieved. Early and late outcomes with Kaplan Meier curves. *STEMI: ST-elevation myocardial infarction; TIMI: Thrombolysis in myocardial infarction.
Keywords
STEMI; Shock cardiogenic; Percutaneous coronary intervention
Abbreviations
CAG: Coronary Angiogram; CS: Conventional Stenting; DBDS: Deflated Balloon-facilitated Direct Stenting; DES: Drug-eluting Stent; DS: Direct Stenting; IABP: Intra-aortic Balloon Pump; IV: Intravenous; MACE: Major Adverse Cardiac Events; MBG: Myocardial Blush Grade; MI: Myocardial Infarction; PCI: Percutaneous Coronary Intervention; PPCI: Primary Percutaneous Coronary Intervention; ST: Stent Thrombosis; STEMI: ST-segment Elevation Myocardial Infarction; TIMI: Thrombolysis in Myocardial Infarction; TLR: Target Lesion Revascularization; TTC: Thrombectomy Trialists Collaboration; TVR: Target Vessel Revascularization.
Central Message
The impact of direct stenting (DS) in comparison to conventional stenting in the management of patients present with acute ST-elevation acute coronary syndrome has been investigated by various meta-analyses and randomized clinical trials which reported conflicting results. The present retrospective observational study demonstrates the safety of DS in ST-segment elevation myocardial infarction (STEMI) patients, without increased risk of restenosis or ST-elevation. However, the DS method is associated with various drawbacks such as vessel visualization requirements, stent-related issues and the need for precise lesion crossing which can be overcome by using a direct-like stenting approach. Further, refinement of patient selection for DS can be used to achieve better clinical outcomes with DS in patients with acute STEMI.
Introduction
In patients present with non-ST-elevation acute coronary syndrome, significant hemodynamic changes occur [1] due to atherosclerotic plaque rupture or erosion, leading to coronary artery occlusion and ST-segment elevation myocardial infarction (STEMI). Primary percutaneous coronary intervention (PPCI) is the preferred reperfusion strategy for acute STEMI management [2], aiming to restore artery patency and achieve microvascular perfusion. Despite its effectiveness, PPCI's success can be hindered by distal embolization and coronary microvascular obstruction in approximately 15.2% of cases, often due to plaque rupture [3] or intervention-related fragmentation.
This limitation has led to the exploration of manual thrombectomy as a method to reduce thrombus burden and distal embolization during PPCI [4-6], potentially improving blood flow and STEMI resolution. However, the Thrombectomy versus PCI alone (TOTAL) trial indicated that thrombectomy before percutaneous coronary intervention (PCI) did not significantly reduce the risk of cardiogenic shock, recurrent myocardial infarction (MI), or cardiovascular death compared to PCI alone and was associated with a higher stroke risk within 30 days [7]. Direct stenting (DS) has emerged as an effective technique for STEMI patients undergoing PCI, offering advantages over conventional stenting (CS) such as a lower risk of distal embolization. Advancements in stent design and manufacturing have supported DS’s adoption in 30%-40% of PCIs [8] demonstrated superior outcomes in restenosis, target lesion revascularization (TLR), death, and MI reduction, attributed to better endothelium preservation and lesion-centered stent positioning. Clinical evidence suggests DS may lead to fewer major adverse cardiac events (MACEs) and improved survival rates compared to CS [9,10], though findings are mixed and call for further investigation. The contrasting results between DS and CS, alongside the limited clinical benefit of thrombectomy, highlight the complexity of treating STEMI and the need for large-scale randomized trials to clarify these interventions’ efficacy. As the medical community continues to debate the optimal approach for revascularization in STEMI patients, integrating clinical outcomes with procedural techniques remains crucial. Future research should aim to refine patient selection for DS, thrombectomy, and CS, considering the intricate dynamics of myocardial viability, thrombus burden, and coronary anatomy to enhance treatment strategies and patient outcomes in acute coronary syndrome management.
Limitations of reperfusion after PCI
PPCI effectively reopens coronary arteries in over 90% of acute STEMI patients, achieving thrombolysis in myocardial infarction (TIMI) grade-3 flow. However, challenges like distal embolization of fragmented thrombus, reperfusion injury from regional inflammatory responses [11], and ischemic microvascular damage lead to incomplete tissue reperfusion, diminished myocardial salvage, and poorer clinical outcomes. Additionally, 9%-15% of PPCI patients experience terminal branch closure due to distal embolization, significantly increasing the 5-year mortality rate by eightfold, highlighting the need for various therapeutic strategies to address these complications and improve patient survival.
Direct stenting versus balloon predilation (conventional stenting)
Despite advancements in stent design and interventional techniques, the decision between DS and balloon predilation remains contentious. Operators may prefer DS for thrombotic lesions to minimize distal embolization and the no-reflow phenomenon, choosing predilatation only when necessary for clear vessel visualization. DS is favored by experienced operators for noncomplex, noncalcified lesions, whereas predilatation is commonly used for severely calcified lesions. The effectiveness of DS over CS in improving clinical outcomes is still debated [12], with studies showing mixed results. Comprehensive randomized clinical studies and meta-analyses are essential to determine the relative efficacy of DS versus CS and other stenting techniques. Table 1A represents the various meta-analysis studies and table 1B represents various randomized clinical studies conducted to compare clinical outcomes of DS and CS.
|
Table 1A: Meta-analysis conducted to compare DS with CS. |
||||
|
Type of study |
No. of studies involved |
Major objective of study |
Primary endpoint of study |
Conclusion |
|
Meta-analysis |
7 studies (10,900 patients) |
To assess the safety and effectiveness of DS with DES implantation |
Primary endpoint was composite of MACE, including MI, repeat revascularization. |
Findings of study suggested that in selected group of patients, DS with DES implantation was safe and may be associated with better clinical outcomes [13]. |
|
Meta-analysis of randomized controlled trials |
5 studies (754 patients) |
In patients present with AMI, to compare clinical outcomes of DS and CS |
Death from cardiovascular causes |
Study findings proposed that ST resolution and clinical outcomes may be improved by DS during PCI in patients present with AMI. To confirm the clinical benefit, further large scale randomized clinical trials should be conducted [14] |
|
Meta-analysis randomized clinical trials) |
24 studies (6803 patients) |
In selected coronary lesions, to compare the clinical outcomes of DS with CS |
Death or MI |
In patients undergoing PCI, clinical outcomes were improved by DS majorly reducing incidence of MI specifically in selected coronary lesions, as suggested by findings of study [15]. |
|
Meta-analysis |
12 studies (8998 patients) |
In patients present with ACS, to compare the efficacy of DS with CS |
Incidence of MACE |
DS method is highly feasible and safe method as it not only reduces incidence of short-term and 1-year mortality but also reduced occurrence of after procedural no-reflow phenomenon in selected patients present with ACS, as demonstrated by study findings [16]. |
|
Table 1B: Randomized clinical trials conducted to compare DS vs CS. |
||||
|
Type of study |
No. of patients enrolled |
Objective of the study |
Primary end point of study |
Conclusion |
|
Multicentre randomized clinical study |
DS(N=173) CS(N=165) |
To assess the long-term clinical outcomes of DS in patients present with MI |
Outcomes, feasibility, and safety of DS |
In the findings of the study, marked difference in term of the need for new target revascularization was not seen in between DS and CS. Furthermore, risk of restenosis was not reduced by DS [14]. |
|
Multicentre-randomized study (HORIZONS-AMI) |
DS(N=698) CS(N=1830) |
To assess the 1-year clinical outcomes of DS in large group of patients who underwent PCI in patients present with STEMI |
Stent arm |
In comparison to implantation of stent after predilation, in treatment of selected lesions in patients present with STEMI, DS is quite safer and effective method, as suggested by findings of the study [17]. |
|
Randomized clinical trial |
DS(N=25) CS(N=25)217 |
To compare DS clinical benefits with CS on immediate blood flow to coronary arteries and short-term clinical outcomes in patients present with AMI |
TIMI-3 flow rate |
In the findings of study, DS provide efficient coronary blood flow in comparison to the CS in patients present with AMI [18]. |
|
Randomized clinical trial |
DS(N=110) CS(N=107) |
To compare safety and efficacy of DS vs CS in AMI patients |
In-hospital major cardiac adverse events |
Significant difference between clinical outcomes was not seen in between DS and CS at the follow-up of 5 years. Furthermore, epicardial and myocardial reperfusion indexes was not improved by DS [19]. |
|
Randomized clinical trial |
DS(N=621) CS(N=1371) |
In patients present with AMI treated with PCI, to compare the clinical impact of DS and CS on in-hospital outcomes and procedural success. |
TIMI-3 flow rate |
In comparison to the patients treated with CS, rate of incidence of advanced heart-failure and in- hospital mortality was significantly lower in DS patients Group, as proposed by findings of the study [20]. |
|
Randomized single-centre trial |
DS(N=102) CS(N=104) |
To demonstrate, that if DS might be able to decline the risk of cardiovascular adverse events associated with implantation of stent during primary angioplasty in patients present with AMI and to compare the outcomes with CS |
Angiographic results, composite end-point of no-reflow phenomenon or distal embolization |
Findings of study demonstrated that DS can be applied effectively and safely in patients present with AMI as it results in marked reduction of microvascular injury [21]. |
|
Randomized clinical study |
DS(N=450) CS(N=356) |
To evaluate long-term clinical outcomes of DS on patients present with STEMI. |
Major adverse events |
Reduction in mid-term MACE rate and all-cause mortality rate was seen in patients treated with DS at the follow-up of 15 years [22]. |
|
Randomized LIPSIA conditioning trial |
DS(N=171) CS(N=171) |
To compare the clinical effect of DS on myocardial injury in comparison to CS in patients with acute reperfused STEMI |
Improvements in Left ventricle parameters |
In the findings of study, marked reduction in the infract size, significant improvement in microvascular obstruction and left ventricular improvements was reported in patients treated with DS in compared to CS [14]. |
|
Randomized pilot study |
DS(N=65) CS(N=65) |
In ACS related lesions, to compare the incidence of no-reflow phenomenon after DS vs CS |
Incidence of no-reflow phenomenon |
In between both groups, marked difference in incidence of no-reflow phenomenon was not seen [23]. |
|
Prospective study |
N=194 DS(n=85) CS(N=121) |
In patients present with ACS undergoing PCI, to compare angiographic, clinical, and procedural outcomes of DS and CS |
Reference luminal diameter |
No marked difference between the two groups (DS vs. CS) was seen in terms of incidence of MACEs [24]. |
|
Single centre prospective study |
DS(N=58) CS(N=30) |
In patients present with STEMI, to compare the long-term and short-term outcomes of DS vs CS. |
Reduction of residual stenosis diameter |
Findings of study demonstrated that follow-up incidence of MACE, clinical and angiographic results were quite similar in both groups [25]. |
|
Observational multi-centre study |
DS(N=489) CS(N=1073) |
In patients present with STEMI undergoing PCI, to compare the effect of DS vs CS on one-year mortality. |
One-year mortality |
Results of study provide evidence that DS is independent predictor of improved one-year survival rate in comparison to DS [26]. |
|
Reterospective Multicentre Observational Study |
DS(N=202) CS(N=414) |
To compare the clinical impact of DS VS CS on small vessel coronary disease in patients undergoing PPCI for STEMI |
Incidence of MACE at follow-up of 2-years |
In comparison to CS, DS implantation was noticed with greater rate of post-procedural TIMI grade-3 flow [27]. |
Table 1: Meta-analysis conducted to compare DS with CS are mentioned in table 1A and randomized clinical trials conducted to compare DS vs CS are mentioned in table 1B. *DS: Direct Stenting; CS: Conventional Stenting.
Deflated balloon-facilitated direct stenting (DBDS) technique to facilitates direct stenting
Culprit lesion length and downstream artery diameter are pivotal for DS during STEMI treatment, yet DS's feasibility often remains low (30% to 50%) due to poor TIMI flow (<1) even after wire placement. To improve DS visualization in totally occluded arteries, a technique using a DBDS has been explored. A single-center study involving 540 STEMI patients undergoing PPCI evaluated DBDS's feasibility, efficacy, and safety. Results indicate that DBDS is a cost-effective, safe method with superior clinical outcomes, suggesting it is a viable alternative to CS and aspiration thrombectomy for treating occluded coronary arteries, enhancing the DS effect in challenging cases [28].
Manual thrombectomy to facilitate DS
Manual thrombectomy has become a prominent adjunctive therapy in acute MI treatment, aiming to reduce thrombus burden during PCI in STEMI patients. Its adoption into routine clinical practice was spurred by early clinical trial successes, showing its potential to diminish stent embolization and enhance outcomes by clearing thrombus before stent deployment [29]. Despite this, the definitive clinical benefits of routine intracoronary thrombus aspiration remain ambiguous. The thrombus aspiration during percutaneous coronary intervention in acute myocardial infarction study (TAPAS) [30] explored this by enrolling 1,071 patients in a single-center, prospective, randomized trial. Patients were divided between conventional PCI and thrombus aspiration groups, with subsequent treatments varying within each group. Thrombus aspiration demonstrated an improvement in myocardial blush grade (MBG) and reduced cardiac death at 1-year follow-up compared to conventional PCI, suggesting its efficacy in improving clinical outcomes post-PCI for STEMI across different patient profiles. Further evidence from the larger thrombus aspiration in STEMI in Scandinavia TASTE [31] and TOTAL [7] trials investigated thrombus aspiration's impact on mortality and procedural success. TASTE, involving 7,259 patients, showed no significant difference in mortality or stroke/neurological complications between thrombus aspiration and PCI-only groups.
Conversely, TOTAL, with 10,732 STEMI patients, found that manual thrombectomy did not notably reduce mortality rates, although DS was more frequent in the thrombectomy group, highlighting DS’s efficiency in lesion crossing on the first attempt. These findings underscore the complexity of determining the optimal treatment strategy for STEMI, reflecting the nuanced interplay between procedural techniques and patient outcomes. Despite varying results, these trials collectively indicate the need for a more tailored approach to incorporating thrombus aspiration and DS in STEMI management, emphasizing the importance of ongoing research and randomized studies to refine clinical protocols and improve patient care in this challenging condition.
Materials and Methods
Design of study
This observational, retrospective study was conducted at the author’s hospital in Bathinda, India, involving 514 patients who underwent PPCI with stenting from September 2018 to February 2023. Participants were primarily over 18, experiencing ongoing ischemia for 12 to 24 hours, with STEMI symptoms starting within 12 hours.
Patients presenting with acute STEMI were the highlight of this study. All-comer patients, including those in cardiogenic shock, were enrolled. DS was the main strategy, with direct-like stenting used as an alternative involving thrombosuction or a preliminary path creation for stent placement. Exclusion criteria included aspirin and ticagrelor contraindications, pregnancy, heavily calcified or tortuous vessels, and missing data. The study, focusing on routine treatment, did not require ethical committee approval.
Protocol of study
In PPCI, in patients present with a TIMI flow of ≥ 1 initially or during post-wire insertion, DS was applied. When DS was not feasible, alternatives such as small balloon ballooning or thrombosuction preceded the stenting, referred to as direct-like stenting. The aim was to minimize clot disturbance and direct stent deployment. Thrombosuction was sometimes necessary to visualize the distal landing zone. Successful DS was defined by <30% residual stenosis and a coronary TIMI flow grade-3 at the procedure end. The study presents four clinical cases, as illustrated in Figures 2, 3, 4 and 5.
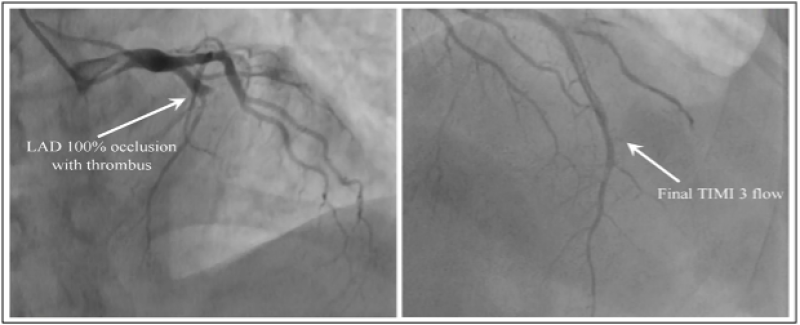
Figure 2 (A-B): Pre and Post PCI CAG after DS demonstrated by Case 1. 2A: In LAD (arrow), presence of 100% lesion with high grade clot burden. 2B: Post PCI CAG (arrow) to demonstrate TIMI grade-3 flow. *PCI: Percutaneous coronary intervention; CAG: Coronary angiogram; DS: Direct stenting; LAD: Left anterior descending artery; TIMI: Thrombolysis in myocardial infarction.
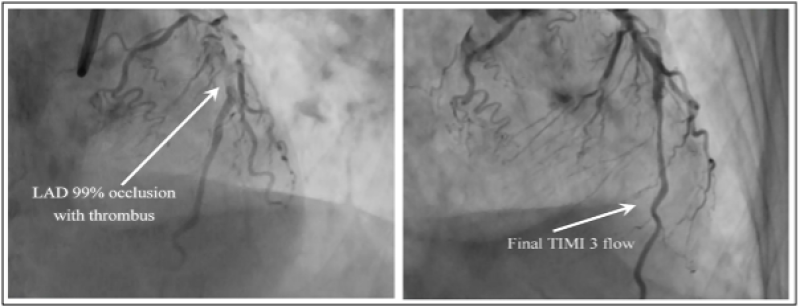
Figure 3 (A-B): Pre and Post PCI CAG demonstrated by Case 2. 3A: CAG (arrow) to demonstrate 98% stenosis present in LAD (proximal). 3B: Post PCI CAG to demonstrate TIMI grade-3 flow (arrow). *PCI: Percutaneous coronary intervention; CAG: Coronary angiogram; LAD: Left anterior descending artery; TIMI: Thrombolysis in myocardial infarction.
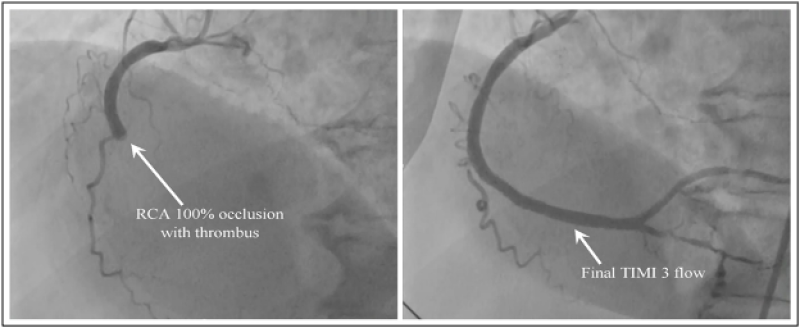
Figure 4 (A-B): Pre and post PCI CAG after DS demonstrated by Case 3. 4A: CAG (arrow) to demonstrate complete occlusion with high grade clot present in proximal RCA. 4B: Post PCI CAG (arrow) to demonstrate TIMI grade-3 flow. *PCI: Percutaneous coronary intervention; CAG: Coronary angiogram; DS: Direct stenting; RCA: Right coronary artery; TIMI: Thrombolysis in myocardial infarction.
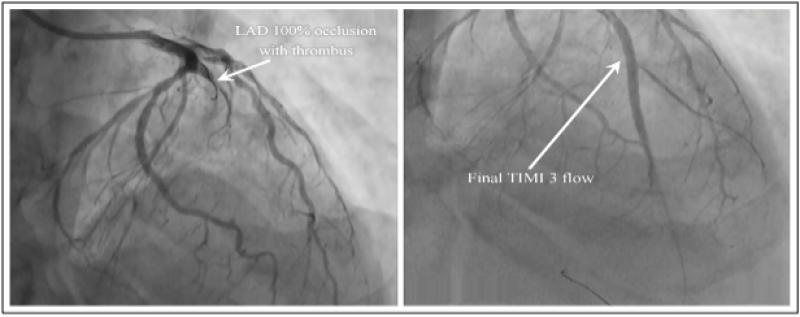
Figure 5 (A-B): Pre and Post PCI CAG demonstrated by Case 4. 5A: CAG (arrow) to demonstrate 100% lesion with high grade clot in LAD. 5B: Post PCI CAG (arrow) to demonstrate TIMI grade-3 flow. *PCI: Percutaneous coronary intervention; CAG: Coronary angiogram; DS: Direct stenting; LAD: Left anterior descending artery; TIMI: Thrombolysis in myocardial infarction.
What authors studied
Between September 2018 and February 2023, 514 STEMI patients received drug-eluting stent (DES) implantation through DS or direct-like stenting in a study. Patients were divided based on hemodynamic stability and the presence of cardiogenic shock. The methods of stent deployment included DS in 367 patients, DS with prior thrombosuction in 51, and direct-like stenting via pre-dilatation plus thrombosuction in 20 or pre-dilatation alone in 66 patients. Post-PCI angiographic evaluations were conducted, and clinical outcomes such as target vessel revascularization (TVR), cardiac death, and MI were monitored during follow-ups at 1, 2, 3 months, and 2-years.
Data collection
Patient demographics, procedural details and patients were used for the collection of data in a retrospective manner. Two expert interventional cardiologists were involved in the evaluation of post-procedure, burden of thrombus, calcification state, and TIMI flow grade. Data on procedural time, contrast media volume, and procedural time was provided by the coronary angiogram (CAG) laboratory.
To collect data on various clinical and in-hospital outcomes, electronic media records, registry databases, and phone calls were used. Routine angiography was not conducted unless clinically indicated during follow-up but was conducted within the 2-years post-PCI period if warranted.
Study endpoints and definitions
During the follow-up, MACEs such as TVR, TLR, MI, or definite stent thrombosis (ST) were primary endpoints. Secondary endpoints included in-hospital death and TLR. TVR covered both PCI and bypass grafting of the initially treated vessel, while TLR involved additional interventions near the original stent.
MI definitions adhered to the latest guidelines [6], and ST followed academic research consortium criteria [13]. Total ischemic time was marked from chest pain onset to initial balloon inflation, with the TIMI system classifying thrombus burden and procedural complications like edge dissection and no-reflow noted.
Results
Execution in the patients who were in cardiogenic shock, the insertion of an Intra-aortic balloon pump (IABP) was done to stabilize the patients. 138 patients out of 147 required IABP. Patients demonstrating clinical hypotension received simultaneous stabilization with intravenous (IV) inotropic support.
The diagnosis was confirmed by using two methods, Echocardiogram and an electrocardiogram followed by performing urgent angiography promptly. Most PCIs were performed using the radial approach after coronary artery angiography. Before PCI, 300 mg of aspirin was administered to patients and 180 mg of ticagrelor, along with an IV bolus of heparin (70 units/kg) and tirofiban (25 ug/kg). At 0.15ug/kg dose, Tirofiban was continued through IV infusion if it was well tolerated by patients.
The procedural steps included the following: DS was performed directly in 372 patients. In 66 patients, 1.5-mm balloon predilation was performed to prepare for DES, followed by its deployment. For 51 patients, thrombosuction with DS was conducted using an aspiration catheter before deploying DES. In 25 patients, thrombosuction was followed by predilatation using a 1.5-mm balloon, followed by direct DES deployment.
The study included 367 (71.40%) patients with normal hemodynamics, averaging 61.0 ± 11 years, and 147 (28.60%) patients with cardiogenic shock, averaging 64.1 ± 11.5 years. The prevalence of diabetes, hypertension, hyperlipidemia, previous MI, and previous PCI among these patients is detailed in Table 2A.
No-reflow phenomenon and edge dissection were the procedural complications seen in 20 (14.5%) and 28 (7.4%) of patients presenting with hemodynamics which is normal and shock (cardiogenic), respectively. The distribution of grades of TIMI flow is shown in Table 2B. In Table 3, details of the hemodynamic characteristics of patients in cardiogenic shock are shown. In 494 (96%) patients, treatment of the target lesion was done successfully. In five lesions (1%), minor dissections were reported. Instances of coronary perforation were not documented. The no-reflow phenomenon was observed in 3% of cases, with 86% achieving TIMI 3 flow. In 78% of cases, MBG was attained, but only in two patients, major Cath complications were reported. Angiographic success was noted in 96% of the 514 treated lesions. In-hospital death, MACE, and TVR were considered a secondary endpoint which were recorded in specific proportions, as detailed in Table 4. Kaplan-Meier curves for MACE and survival are depicted in Figures 6 and 7.
|
Table 2A: Clinical characteristics and baseline demographics. |
||
|
Serials |
STEMI+Normal blood pressure |
STEMI+shock |
|
Total pts(%) |
367(71.40) |
147(28.60) |
|
Age in years, mean ± |
61 ± 11 |
64.1 ± 11.5 |
|
Smoker, n(%) |
187(50.95) |
67(45.58) |
|
EF, (%) mean ± |
46.2 ± 8.3 |
37.3 ± 12 |
|
Diabetes mellitus, n(%) |
154(41.96) |
53(36.05) |
|
Hypertension, n(%) |
175(47.68) |
61(41.50) |
|
Hyperlipidemia, n(%) |
65(17.71) |
33(22.44) |
|
Prior MI, n(%) |
38(10.35) |
25(17.00) |
|
Prior PCI, n(%) |
39(10.63) |
9(6.12) |
|
Prior CABG, n(%) |
5(1.36) |
5(3.40) |
|
Renal insufficiency, n(%) |
45(12.26) |
23(15.65) |
|
Ischemia time in minutes mean ± |
114.2 ± 41.8 |
116.1 ± 38.1 |
|
Table 2B: Characteristics of lesion. |
||
|
Parameter |
STEMI+Normal blood pressure (n=367) |
STEMI+shock (n=147) |
|
Culprit artery: |
||
|
LAD |
105(28.61) |
57(38.76) |
|
LCx |
27(7.36) |
24(16.33) |
|
RCA |
205(55.86) |
58(39.46) |
|
Other |
24(6.54) |
18(12.24) |
|
Procedural complication: |
26(7.08) |
22(14.97) |
|
Edge dissection |
13(3.54) |
7(4.76) |
|
No reflow |
16(4.36) |
12(8.16) |
|
Procedural characteristics: |
||
|
Post-dilation |
52(14.17) |
14(9.52) |
|
Aspiration thrombectomy |
24(6.54) |
6(4.08) |
|
Length of stent, mean [mm] |
17.8 ± 6.18 |
22.92 ± 6.66 |
|
Diameter of stent mean [mm] |
2.73 ± 0.24 |
2.28 ± 0.13 |
|
Time of procedure mean [min] |
47.0 ± 13.1 |
46.1 ± 16.5 |
|
Time of fluoroscopy in minutes, mean ± |
8.4 ± 5.2 |
14.1 ± 6.7 |
|
Contrast volume, [ml] mean ± |
124.1 ± 50.2 |
151.4 ± 86.1 |
|
Postprocedural TIMI flow III, (%) |
350(95.37) |
128(87.07) |
|
Lesion location: |
||
|
Proximal |
115(31.33) |
53(36.05) |
|
Mid |
210 |
61 |
|
Distal |
54(14.71) |
21(14.29) |
|
Multiple stents, n |
78(21.25) |
32(21.77) |
|
Multivessel disease, (%) n |
51(13.87) |
27(18.37) |
|
Calcified stenosis, (%) n |
54(14.71) |
20(13.61) |
|
Thrombus burden: |
||
|
Low thrombus burden |
206(56.13) |
53(36.06) |
|
High thrombus burden |
168(45.78) |
91(61.90) |
|
Glycoprotein IIb/IIIa inhibitors |
296(80.65) |
118(80.27) |
|
Bifurcation stenting |
9(2.45) |
1(0.68) |
|
Thrombectomy |
57(15.53) |
19(12.93) |
|
TIMI flow at baseline |
||
|
0 |
215(58.58) |
80(54.42) |
|
1 |
95(25.89) |
36(24.49) |
|
2 |
32(8.72) |
9(6.12) |
|
3 |
36(9.81) |
15(10.20) |
|
Final TIMI flow 3 |
315(85.83) |
125(85.03) |
|
No reflow |
1(0.27) |
2(1.36) |
|
Dissection |
7(1.91) |
3(2.04) |
|
Distal embolization |
18(4.90) |
5(3.40) |
|
Average contrast volume used |
55 ± 25 |
35 ± 5 |
|
IABP use |
Nil |
133(90.48) |
Table 2: Clinical characteristics and baseline demographics are demonstrated in table 2A and characteristics of lesion are demonstrated in table 2B.
|
Patients in shock (n=147) |
|
|
Stages of shock according to SCAI classification |
46 patients in stage C, D and E |
|
SP (Mean) |
70 ± 16 mm of Hg |
|
DP (Mean) |
54 ± 10 mm of Hg |
|
MP (Mean) |
60 ± 18 mm of Hg |
|
PAP (Mean) |
18/9 ± 21/16 mm of Hg |
|
PAPI (Mean) |
<1.2 in more than 19 patients |
|
CO (Mean) |
4 ± 1.8 L/min |
|
Number of inotropic agents used |
>3(80%) |
|
IABP |
(138) 94% |
Table 3: Details of hemodynamics in shock patients.
|
In-hospital outcomes |
||
|
Variables |
STEMI+Normal blood pressure (n=367) |
STEMI+shock (n=147) |
|
MI |
6(1.63) |
4(2.72) |
|
TLR |
9(2.45) |
5(3.40) |
|
TVR |
4(1.90) |
3(2.04) |
|
ST |
2(0.54) |
3(2.04) |
|
MACE |
8(2.18) |
3(2.04) |
|
All-cause death |
5(1.36) |
26(17.69) |
|
At 1-month on follow, clinical outcomes |
||
|
Variables |
STEMI+Normal blood pressure (n=367) |
STEMI+shock (n=147) |
|
MI |
5(1.36) |
4(2.72) |
|
TLR |
2(0.54) |
2(1.36) |
|
TVR |
6(1.63) |
3(2.04) |
|
ST |
2(0.54) |
3(2.04) |
|
MACE |
10(2.72) |
5(3.40) |
|
All-cause death |
1(0.27) |
30(20.41) |
|
At 1-year on follow, Clinical outcomes |
||
|
Variables |
STEMI+Normal blood pressure (n=367) |
STEMI+shock (n=147) |
|
MI |
10(2.72) |
8(5.44) |
|
TLR |
7(1.91) |
3(2.04) |
|
TVR |
11(3.00) |
4(2.72) |
|
ST |
1(0.27) |
4(2.72) |
|
MACE |
18(4.90) |
9(6.12) |
|
All-cause death |
6(1.63) |
35(23.81) |
|
At 2-years on follow, Clinical outcomes |
||
|
Variables |
STEMI+Normal blood pressure (n=367) |
STEMI+shock (n=147) |
|
MI |
19(5.18) |
11(7.48) |
|
TLR |
9(2.45) |
10(6.80) |
|
TVR |
14(3.81) |
7(4.76) |
|
ST |
1(0.2) |
5(3.40) |
|
MACE |
42(11.44) |
28(19.05) |
|
All-cause death |
9(2.45) |
40(27.21) |
Table 4: At the follow-up of 1-month, 1-year and 2-years, various In-hospital clinical outcomes.
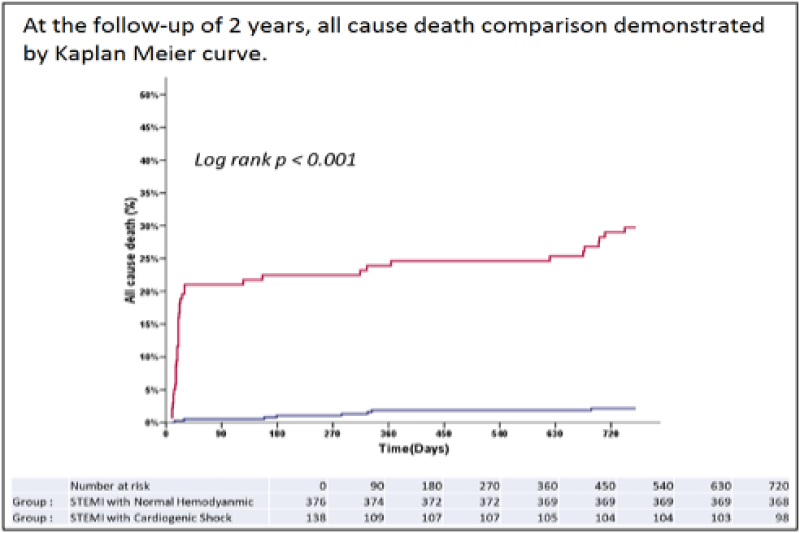
Figure 6: At the follow-up of 2 years, all cause death comparison demonstrated by kaplan meier curve.
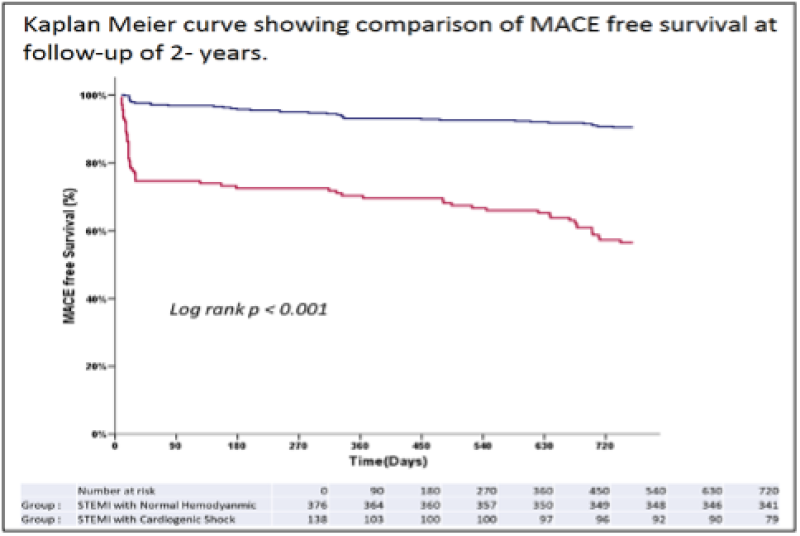
Figure 7: Kaplan meier curve showing comparison of MACE free survival at follow-up of 2-years. *MACE: Major adverse cardiac events.
Discussion
PCI is the preferred reperfusion strategy for STEMI patients, with DS, recognized for its role in minimizing distal embolization and microvascular obstruction. Despite its effectiveness, DS lacks established guidelines [32] and is not universally feasible, prompting a retrospective analysis of STEMI patients undergoing primary angioplasty.
This analysis distinguished between patients with normal hemodynamics and those with cardiogenic shock, focusing on DS utilization. The integration of DS with thrombus aspiration has been explored in significant trials such as TAPAS [5], TASTE [31], and TOTAL [7] with the Thrombectomy Trialists Collaboration (TTC) [33] offering the largest observational dataset.
The TTC study showed that DS post-aspiration thrombectomy, utilized in about 32% of cases, reduced contrast use and fluoroscopy time, although it didn't conclusively improve clinical outcomes. This suggests that while DS following thromboaspiration is technically feasible and may have procedural benefits, its impact on long-term clinical outcomes remains uncertain, potentially due to the inadvertent distal embolization caused by thrombectomy devices [34].
Follow-up data indicated a decrease in cerebrovascular incidents and cardiovascular mortality rates, alongside a reduction in vessel revascularization after one year. Despite concerns about stent mal-apposition or coverage, the study reported no increased risk, suggesting that DS might reduce the incidence of inadequate ST-segment resolution.
However, the benefits of DS over CS are not definitively clear, as both strategies have been shown to address only a part of the reperfusion challenges in STEMI. With advancements in PCI techniques and adjunctive medications, the gap for clinical improvements via DS in the modern era of low STEMI mortality (<2%) appears narrow [35]. Notably, DS has been associated with fewer instances of the no-reflow phenomenon and improved clinical outcomes, as evidenced by meta-analyses [16] and trials like the LIPSIA CONDITIONING [36], which reported smaller infarct sizes and lower mortality rates with DS compared to CS.
Despite these promising results, the real-world impact of DS on mortality is hard to quantify, especially in high-risk groups like those with cardiogenic shock. The technique's efficacy, especially when employing modifications like deflated balloon-assisted stenting for high-grade thrombus cases, underscores its potential as a safe and effective strategy in STEMI management. Emerging research suggests DS could lead to reduced long-term mortality [37], even though some studies report no significant outcomes difference between DS and CS [14,19,38,39]. This discrepancy highlights the need for larger, more focused studies, particularly involving patients with severe complications. The Swedish Coronary Angiography and Angioplasty Registry's [40] findings on reduced restenosis rates and ST due to newer DES generations further encourage the pursuit of DS, albeit the necessity for more extensive research remains, particularly to understand its full potential in complex STEMI cases.
Study limitations
Various inherent limitations are present in this single-centre retrospective observational study. Key variables such as left ventricular ejection fraction, stent length, and diameter weren't analyzed. The Incidence of the Left interior descending infract-related artery which can have a huge impact on the clinical outcomes in the CS strategy, wasn’t taken into consideration. Another major limitation is the exclusive use of first-generation DES. Moreover, the only thrombus aspiration system utilized was rheloytic thrombectomy. As an observational study not comparing CS, propensity score-based methods were not applicable.
Importance of the study
• DS and direct-like stenting are often successful and can be employed when CS methods are impractical.
• These methods use less contrast and typically encounter fewer complications during surgery. Most patients experience improved TIMI-3 flow and reduced distal embolization.
• While more effectiveness is seen with DS or direct-like stenting without thrombectomy, a well-conducted randomized controlled trial is needed to verify this
• In essence, this study and the ensuing discussions recommend prioritizing direct approaches in primary PCI for patients with STEMI.
• Compared with the GUSTO-2 trial, te authors nonrandomized study showed a total MACE of 14%, including a 5.7% mortality rate, 3.9 % reinfarction rate, and 0.9 % stroke rate. The author’s outcomes, with a 2.1% mortality, 4.9 % reinfarction, and a 7.4% MACE rate, are comparatively favorable.
• A randomized controlled trial comparing the clinical outcomes of conventional methods with direct or direct-like stenting for primary PCI would be advisable. DS in STEMI and observational outcomes are described in a graphical abstract.
Conclusion
In the maximum number of patients present with acute STEMI, DS or direct-like stenting is achievable, even where high thrombus is present. Direct-like stenting is viable when the stents cannot cross, and the distal landing zone is rendered unclear in angiograms. DS or direct-like stenting uses less contrast and typically results in minimal complications during surgery. These methods considerably reduce distal embolization rates and enhance TIMI grade-3 flow. The authors findings underscore the importance of a direct approach to PPCI for patients with STEMI. The non-randomized study findings are encouraging, especially when compared with the outcomes of the GUSTO-2 trial, which showed lower mortality and MACE rates.
Summary points
1. STEMI is a severe manifestation of CAD with high morbidity and mortality rates. Primary treatment involves PCI.
2. While PPCI is effective, suboptimal myocardial reperfusion can occur, leading to the exploration of alternative strategies like CS, DS and manual thrombectomy.
3. DS offers advantages, including reduced procedural time and contrast media usage. Recent studies indicate its safety in STEMI patients, without increased risks of restenosis or ST, often linked to improper stent sizing.
4. DS has drawbacks, such as potential stent-related issues, vessel visualization requirements, and the need for precise lesion crossing. Routine use is limited without clear vessel visualization. These limitations can be overcome somewhat by direct-like stenting in some patients.
5. In acute reperfused MI, DS has demonstrated safety and efficacy compared to CS. It has been associated with reduced heart failure hospitalizations, lower mortality rates, and significant reductions in infarct size, supported by various short-term and long-term studies.
Author contributions
The lead author of the review article is Dr. Rohit Mody. Dr. Abha Bajaj Nee Sheth, Dr. Debabrata Dash, Dr. Bhavya Mody, Dr. Inderjeet Singh Monga, Dr. Lakshay Rastogi Dr. Bankey Bihari, Dr. Rahul Singla and Dr. Amit Munjal had equal and substantial contributions in the formation of this review article. They were involved in conceptualization, data curation, formal analysis, resources, software, validation, visualization, writing-original draft, writing, review and editing.
Acknowledgment
The author thank Mr. Rohit for assisting me to finalize the review article. The figures are edited by Jiwan Singh.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Ethical approval was not required since it is an accepted procedure.
Consent for publication
Written consent has been obtained to publish the review article from the guardian. The consent copy is available with the authors and ready to be submitted if required.
References
1. Knežević B, Nikolić G, Dragnić S, Musić L, Bošković A. Successful Treatment of Cardiogenic Shock by Stenting of the Left Main Coronary Artery in Acute Myocardial Infarction. Vojnosanit Pregl. 2008;65(10):769-73. PubMed | CrossRef
2. Simard T, Hibbert B, Ramirez FD, Froeschl M, Chen YX, O'Brien ER. The Evolution of Coronary Stents: A Brief Review. Can J Cardiol. 2014;30(1):35-45. PubMed | CrossRef
3. Henriques JP, Zijlstra F, Ottervanger JP, De Boer MJ, Van'T Hof AW, Hoorntje JC, et al. Incidence and Clinical Significance of Distal Embolization During Primary Angioplasty for Acute Myocardial Infarction. Eur Heart J. 2002;23(14):1112-7. PubMed | CrossRef
4. Topol EJ, Yadav JS. Recognition of the Importance of Embolization in Atherosclerotic Vascular Disease. Circulation. 2000;101(5):570-80. PubMed | CrossRef
5. Burzotta F, Trani C, Romagnoli E, Mazzari MA, Rebuzzi AG, De Vita M, et al. Manual Thrombus-aspiration Improves Myocardial Reperfusion: The Randomized Evaluation of the Effect of Mechanical Reduction of Distal Embolization by Thrombus-aspiration in Primary and Rescue Angioplasty (REMEDIA) Trial. J Am Coll Cardiol. 2005;46(2):371-6. CrossRef
6. Svilaas T, van der Horst IC, Zijlstra F. Thrombus Aspiration During Percutaneous Coronary Intervention in Acute Myocardial Infarction Study (TAPAS)-study Design. Am Heart J. 2006;151(3):597-e1. PubMed | CrossRef
7. Bhindi R, Kajander OA, Jolly SS, Kassam S, Lavi S, Niemelä K, et al. Culprit Lesion Thrombus Burden After Manual Thrombectomy or Percutaneous Coronary Intervention-alone in ST-segment Elevation Myocardial Infarction: The Optical Coherence Tomography Sub-study of the TOTAL (ThrOmbecTomy versus PCI ALone) Trial. Eur Heart J. 2015;36(29):1892-900. PubMed | CrossRef
8. Barbato E, Marco J, Wijns W. Direct Stenting. Eur Heart J. 2003;24(5):394-403. PubMed | CrossRef
9. Silva-Orrego P, Bigi R, Colombo P, De Marco F, Oreglia JA, Klugmann S, et al. Direct Stenting After Thrombus Removal Before Primary Angioplasty in Acute Myocardial Infarction. J Interv Cardiol. 2008;21(4):300-6. PubMed | CrossRef
10. Dudek D, Mielecki W, Burzotta F, Gasior M, Witkowski A, Horvath IG, et al. Thrombus Aspiration Followed by Direct Stenting: A Novel Strategy of Primary Percutaneous Coronary Intervention in ST-segment Elevation Myocardial Infarction. Results of the Polish-Italian-Hungarian Randomized ThrombEctomy Trial (PIHRATE Trial). Am Heart J. 2010;160(5):966-72. PubMed | CrossRef
11. Heusch G. The Coronary Circulation as a Target of Cardioprotection. Circ Res. 2016;118(10):1643-58. PubMed | CrossRef
12. Belardi JA, Albertal M. Direct Stenting Versus Balloon Predilation: Jury is Still Out. Catheter Cardiovasc Interv. 2017;90(2):223-4. PubMed | CrossRef
13. Magalhaes MA, Minha SA, Lhermusier T, Pendyala L, Escarcega RO, Baker NC, et al. Does Direct Stenting with Drug‐eluting Stents Improve Outcome? A Meta‐analysis of 10,900 Patients. Catheter Cardiovasc Interv. 2017;90(2):213-22. PubMed | CrossRef
14. Elbaz M, El Mokhtar E, Khalifé K, Citron B, Izaaz K, Hamon M, et al. Is Direct Coronary Stenting the Best Strategy for Long-term Outcome? Results of the Multicentric Randomized Benefit Evaluation of Direct Coronary Stenting (BET) Study. Am Heart J. 2002;144(4):J1-6. PubMed | CrossRef
15. Piscione F, Piccolo R, Cassese S, Galasso G, D'Andrea C, De Rosa R, et al. Is Direct Stenting Superior to Stenting with Predilation in Patients Treated with Percutaneous Coronary Intervention? Results from a Meta-analysis of 24 Randomised Controlled Trials. Heart. 2010;96(8):588-94. PubMed | CrossRef
16. Li C, Zhang B, Li M, Liu J, Wang L, Liu Y, et al. Comparing Direct Stenting with Conventional Stenting in Patients with Acute Coronary Syndromes: A Meta-analysis of 12 Clinical Trials. Angiology. 2016;67(4):317-25. PubMed | CrossRef
17. Möckel M, Vollert J, Lansky AJ, Witzenbichler B, Guagliumi G, Peruga JZ, et al. Comparison of Direct Stenting with Conventional Stent Implantation in Acute Myocardial Infarction. Am J Cardiol. 2011;108(12):1697-703. PubMed | CrossRef
18. Ozdemir R, Sezgin AT, Barutcu I, Topal E, Gullu H, Acikgoz N. Comparison of Direct Stenting Versus Conventional Stent Implantation on Blood Flow in Patients with ST-segment Elevation Myocardial Infarction. Angiology. 2006;57(4):453-8. PubMed | CrossRef
19. Gasior M, Gierlotka M, Lekston A, Wilczek K, Zebik T, Hawranek M, et al. Comparison of Outcomes of Direct Stenting Versus Stenting after Balloon Predilation in Patients with Acute Myocardial Infarction (DIRAMI). Am J Cardiol. 2007;100(5):798-805. PubMed | CrossRef
20. Isik T, Ayhan E, Uyarel H, Ergelen M, Cicek G, Osmonov D, et al. A Comparison of Direct Versus Conventional Stenting in Patients Undergoing Primary Angioplasty for ST-elevation Myocardial Infarction. Coron Artery Dis. 2012;23(5):348-53. PubMed | CrossRef
21. Loubeyre C, Morice MC, Lefèvre T, Piéchaud JF, Louvard Y, Dumas P. A Randomized Comparison of Direct Stenting with Conventional Stent Implantation in Selected Patients with Acute Myocardial Infarction. J Am Coll Cardiol. 2002;39(1):15-21. PubMed | CrossRef
22. Scarparo P, Improta R, Wilschut J, Kardys I, Den Dekker WK, Daemen J, et al. Very Long-term Clinical Outcomes After Direct Stenting in Patients Presenting with ST-segment Elevation Myocardial Infarction. Cardiovasc Revasc Med. 2022;41:144-50. PubMed | CrossRef
23. Sabatier R, Hamon M, Zhao QM, Burzotta F, Lecluse E, Valette B, et al. Could Direct Stenting Reduce No-reflow in Acute Coronary Syndromes? A Randomized Pilot Study. Am Heart J. 2002;143(6):1027-32. PubMed | CrossRef
24. Süselbeck T, Türkoglu A, Lang S, Krause B, Kralev S, Haghi D, et al. Direct Versus Conventional Stent Implantation in Patients with Acute Coronary Syndrome Just Before the Era of Drug-eluting Stents. Int J Cardiol. 2005;105(1):85-9. PubMed | CrossRef
25. Tacoy G, Yazici GE, Erden M, Timurkaynak T. The Comparison of Early and Late Outcome of Direct and Conventional Stenting of Patients with ST-elevation Myocardial Infarction. Ther Adv Cardiovasc Dis. 2009;3(3):181-6. PubMed | CrossRef
26. McCormick LM, Brown AJ, Ring LS, Gajendragadkar PR, Dockrill SJ, Hansom SP, et al. Direct Stenting is an Independent Predictor of Improved Survival in Patients Undergoing Primary Percutaneous Coronary Intervention for ST Elevation Myocardial Infarction. Eur Heart J Acute Cardiovasc Care. 2014;3(4):340-6. PubMed | CrossRef
27. Cosansu K, Ureyen C, Vatan M, Agac M, Kilic H, Akdemir R. Impact of Direct Stenting on Clinical Outcomes for Small Vessel Coronary Artery Disease in Patients Undergoing Primary Percutaneous Coronary Intervention for ST-elevation Myocardial Infarction. Postepy Kardiol Interwencyjnej. 2019;15(4):404-11. PubMed | CrossRef
28. Verma B, Singh A, Saxena AK, Kumar M. Deflated Balloon-facilitated Direct Stenting in Primary Angioplasty (The DBDS technique): A Pilot Study. Cardiol Res. 2018;9(5):284. PubMed | CrossRef
29. Mamas MA, Fraser D, Fath-Ordoubadi F. The Role of Thrombectomy and Distal Protection Devices During Percutaneous Coronary Interventions. Eurointervention. 2008;4(1):115-23. PubMed | CrossRef
30. Jolly SS, Cairns JA, Yusuf S, Meeks B, Pogue J, Rokoss MJ, et al. Randomized Trial of Primary PCI with or without Routine Manual Thrombectomy. N Engl J Med. 2015;372(15):1389-98. PubMed | CrossRef
31. Fröbert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, et al. Thrombus Aspiration During ST-segment Elevation Myocardial Infarction. N Engl J Med. 2013;369(17):1587-97. PubMed | CrossRef
32. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-segment Elevation: The Task Force for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119-77. PubMed | CrossRef
33. Mahmoud KD, Jolly SS, James S, Džavík V, Cairns JA, Olivecrona GK, et al. Clinical Impact of Direct Stenting and Interaction with Thrombus Aspiration in Patients with ST-segment Elevation Myocardial Infarction Undergoing Percutaneous Coronary Intervention: Thrombectomy Trialists Collaboration. Eur Heart J. 2018;39(26):2472-9. PubMed | CrossRef
34. van't Hof AW, Ottervanger JP. Primary Angioplasty for STEMI: Hard to Improve Upon. Lancet. 2016;387(10034):2166-8. PubMed | CrossRef
35. Neumann FJ, Gick M. Direct Stenting in ST-elevation Myocardials nfarction: Convenient, but not Improving Outcomes. Eur Heart J. 2018;39(26):2480-3. PubMed | CrossRef
36. Yang EH. Should we be More Direct with STEMI Patients? Int J Cardiol. 2019;283:93-4. PubMed | CrossRef
37. Saad M, Stiermaier T, Fuernau G, Pöss J, de Waha-Thiele S, Desch S, et al. Impact of Direct Stenting on Myocardial Injury Assessed by Cardiac Magnetic Resonance Imaging and Prognosis in ST-elevation Myocardial Infarction. Int J Cardiol. 2019;283:88-92. PubMed | CrossRef
38. Caputo RP, Flately M, Ho KK, Baim DS. Safety and Effectiveness of Stent Implantation without Predilation for Small Coronary Arteries. Catheter Cardiovasc Interv. 2003;59(4):455-8. PubMed | CrossRef
39. Brito Jr FS, Caixeta AM, Perin MA, Rati M, Arruda JA, Cantarelli M, et al. Comparison of Direct Stenting Versus Stenting with Predilation for the Treatment of Selected Coronary Narrowings. Am J Cardiol. 2002;89(2):115-20. PubMed | CrossRef
40. Sarno G, Lagerqvist B, Olivecrona G, Varenhorst C, Danielewicz M, Hambraeus K, et al. Real‐life Clinical Outcomes with Everolimus Eluting Platinum Chromium Stent with an Abluminal Biodegradable Polymer in Patients from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Catheter Cardiovasc Interv. 2017;90(6):881-7. PubMed | CrossRef
Rohit Mody1*, Abha Bajaj Nee Sheth2, Debabrata Dash3, Bhavya Mody4, Inderjeet Singh Monga5, Lakshay Rastogi6, Amit Munjal7, Bankey Bihari8 and Rahul Singla9
1MD, DM, Department of Cardiology, Mody Harvard Cardiac Institute and Research Centre, Krishna Super Specialty Hospital, Bathinda, Punjab, India
2MBBS, MS, Department of Anatomy, Doctor Harvansh Singh Judge Institute of Dental Sciences and Hospital, Panjab University, Chandigarh, India
3MD, DM, FICC, FAPSC, FCCP, FSCAI, Department of Cardiology, Aster Hospital, Mankhool, Dubai, Al Quasis, UAE
4MBBS, Department of Medicine, Kasturba Medical College, Manipal, Karnataka, India
5MBBS, DNB, DM, FSCAI, Department of Cardiology, Command Hospital Chandimandir, Panchkula, Haryana, India
6MBBS, Department of Medicine, Kasturba Medical College, Manipal, Karnataka, India
7MBBS, MD, DMB, Department of Cardiology, Doctor Asha Memorial Multispecialty Hospital, Fatehabad, Haryana, India
8MBBS, MD, Department of Medicine, Government Medical College and Rajindra Hospital, Patiala, Punjab, India
9MBBS, USMLE2, Extern, Internal Medicine, MedStar Franklin Square Medical Centre, Baltimore, USA
*Corresponding Author: Rohit Mody, MD, DM, Department of Cardiology, Mody Harvard Cardiac Institute and Research Centre, Krishna Super Specialty Hospital, Bathinda, Punjab, India.
Copyright© 2024 by Mody R, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Mody R, Sheth ABN, Dash D, Mody B, Monga IS, Rastogi L, et al. Direct Stenting in ST-elevation Myocardial Infarction: Comprehensive Review and Implications for Primary PCI Guidelines Inclusion. J Cardiol Cardiovasc Res. 2024;5(1):142-61. DOI: https://doi.org/10.37191/Mapsci-JCCR-5(1)-091
